Welcome to issue 1, 2013, of Organic & Biomolecular Chemistry. The entire issue is free for you to access so get busy downloading!
 Front cover: This very elegant cover highlights the work of Leung Sheh and co-workers. The study of Sheh et al. further explores the allosteric features of DNA–peptide molecular recognition with regard to interaction networks, choosing designed peptides that exhibit significant cooperativity in binding to DNA.
Front cover: This very elegant cover highlights the work of Leung Sheh and co-workers. The study of Sheh et al. further explores the allosteric features of DNA–peptide molecular recognition with regard to interaction networks, choosing designed peptides that exhibit significant cooperativity in binding to DNA.
Energetic studies on DNA–peptide interaction in relation to the enthalpy–entropy compensation paradox
Robin C. K. Yang, Jonathan T. B. Huang, Shih-Chuan Chien, Roy Huang, Kee-Ching G. Jeng, Yen-Chung Chen, Mokai Liao, Jia-Rong Wu, Wei-Kang Hung, Chia-Chun Hung, Yu-Ling Chen, Michael J. Waring and Leung Sheh
 Inside cover: Featuring on this cover is the work of Alison Thompson and co-workers who have structurally modified the C-ring of a series of prodigiosenes to produce a series of analogues that show promising activity against leukemia cell lines.
Inside cover: Featuring on this cover is the work of Alison Thompson and co-workers who have structurally modified the C-ring of a series of prodigiosenes to produce a series of analogues that show promising activity against leukemia cell lines.
Investigations regarding the utility of prodigiosenes to treat leukemia
Deborah A. Smithen, A. Michael Forrester, Dale P. Corkery, Graham Dellaire, Julie Colpitts, Sherri A. McFarland, Jason N. Berman and Alison Thompson
Both of these papers have been highlighted by the reviewers as being HOT.
| Also in this issue:
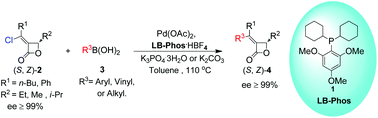
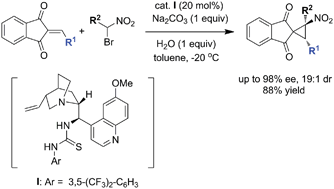
HOT – Pd-catalyzed Suzuki coupling reaction of chloroalkylidene-β-lactones with LB-Phos as the ligand HOT – An efficient organocatalytic enantioselective synthesis of spironitrocyclopropanes HOT – Synthesis of a four-component [3]catenane using three distinct noncovalent interactions Find all this great FREE content and more in issue 1 |











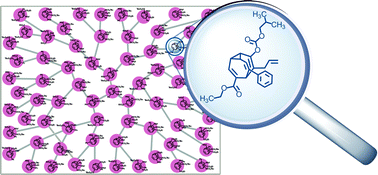
![Synthesis of a four-component [3]catenane using three distinct noncovalent interactions](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB26587G)
![1,5-(H, RO, RS) shift/6π-electrocyclic ring closure tandem processes on N-[(α-heterosubstituted)-2-tolyl]ketenimines](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C2OB27010B)