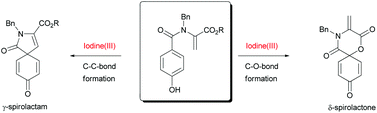
In this HOT communication Boris J. Nachtsheim and colleagues at Eberhard Karls University present preliminary results towards a fast and efficient synthetic approach of γ-spirolactams based on an iodine(III)-mediated oxidative spirocyclization of 2-(4-hydroxybenzamido)-acrylates.
In addition Nachtsheim et al. discuss the discovery of an unexpected side reaction, a rare iodine(III)-mediated spirocyclization that gave δ-spirolactones. With fluorinated solvents and modified reaction conditions this side reaction was able to produce δ-spirolactones in yields up to 70%.
Interested? Find out more about these reactions and their mechanisms by downloading this Communication for FREE.
A hypervalent iodine-mediated spirocyclization of 2-(4-hydroxybenzamido)acrylates – unexpected formation of δ-spirolactones
Christian Hempel, Nicole M. Weckenmann, C. Maichle-Moessmer and Boris J. Nachtsheim
DOI: 10.1039/C2OB26815A