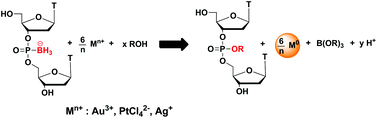
In this HOT communication Marvin H. Caruthers and co-workers from the University of Colorado show that oligodeoxyribonucleotides bearing boranephosphonate linkages (bpDNA) are able to reduce a variety of metal ions whilst producing nanoparticles. Carauthers et al. discovered that during the reduction of metal ions in protic solvents, the B–P bond undergoes solvolysis and generates phosphate diesters (in water) or triesters (in alcohols).
To highlight the utility of this reaction Caruthers et al. synthesised an oligomer containing an O-methyl phosphate triester linkage, which cannot be prepared via usual methods of DNA synthesis as such triesters are base labile.
Caruthers et al. believe that, in addition to bpDNA‘s potential applications in the construction of DNA templated metallic nanostructures, the new reactivity of boranephosphonates shown can be exploited as a general method for the introduction of a variety of functionalities onto the DNA backbone via phosphate triesters.
Intrigued? Read this Communication for free for the next 4 weeks to find out more!
This Communication is part of the Nucleic acids: new life, new materials collection. Why not take a look at similar work and let us know what you think here on the blog or on Twitter.
Reduction of metal ions by boranephosphonate DNA
Subhadeep Roy, Magdalena Olesiak, Petra Padar, Heather McCuen and Marvin H. Caruthers
DOI: 10.1039/C2OB26661J