Guess what…. yes you got it, it’s Organic & Biomolecular Chemistry time! This week’s issue has 1 Perspective, 5 Communications and 17 Papers for your reading pleasure.
 Front cover:
Front cover:
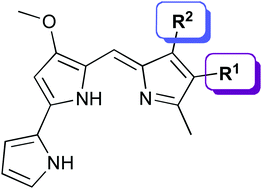
Put your hands together for this very nice image highlighting the work of David B. Amabilino and colleagues. Amabilino et al. use the induction of chirality in achiral aggregates of an oligo(p-phenylenevinylene) to detect the enantiomeric excess in acids used in the resolution of chiral compounds. These achiral supramolecular stacks can detect the enantiomeric excess of substoichiometric amounts different organic acids.
Sensitive detection of enantiomeric excess in different acids through chiral induction in an oligo(p-phenylenevinylene) aggregate
François Riobé, Albertus P. H. J. Schenning and David B. Amabilino
DOI: 10.1039/C2OB26411K
 Inside cover:
Inside cover:
This image of seaside chemistry is courtesy of Ming-Hua Xu and co-workers at Shanghai Institute of Materia Medica. Xu et al. present their work on developing a highly efficient and enantioselective route to quaternary carbon-containing heteroaromatic α-hydroxy esters. Xu et al. employ the catalytic asymmetric 1,2-addition of arylboronic acids to obtain these heteroaryl α-ketoesters.
Rhodium-catalyzed enantioselective 1,2-addition of arylboronic acids to heteroaryl α-ketoesters for synthesis of heteroaromatic α-hydroxy esters
Hui Wang, Ting-Shun Zhu and Ming-Hua Xu
DOI: 10.1039/C2OB26316E
Hot articles in this issue:
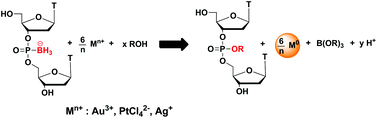
Reduction of metal ions by boranephosphonate DNA
Subhadeep Roy, Magdalena Olesiak, Petra Padar, Heather McCuen and Marvin H. Caruthers
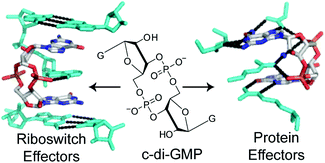
The bacterial second messenger c-di-GMP: probing interactions with protein and RNA binding partners using cyclic dinucleotide analogs
Carly A. Shanahan and Scott A. Strobel
As always the articles featured on the covers are free to access for 6 weeks, and the HOT articles are free for 4!
For all this and much, much more take a look at the issue online today!