This month sees the following articles in Lab on a Chip that are in the top ten most accessed:-
Quantitative and sensitive detection of rare mutations using droplet-based microfluidics
Deniz Pekin, Yousr Skhiri, Jean-Christophe Baret, Delphine Le Corre, Linas Mazutis, Chaouki Ben Salem, Florian Millot, Abdeslam El Harrak, J. Brian Hutchison, Jonathan W. Larson, Darren R. Link, Pierre Laurent-Puig, Andrew D. Griffiths and Valérie Taly
Lab Chip, 2011, 11, 2156-2166
DOI: 10.1039/C1LC20128J
Paper-based piezoresistive MEMS sensors
Xinyu Liu, Martin Mwangi, XiuJun Li, Michael O’Brien and George M. Whitesides
Lab Chip, 2011, 11, 2189-2196
DOI: 10.1039/C1LC20161A
Multiplex digital PCR: breaking the one target per color barrier of quantitative PCR
Qun Zhong, Smiti Bhattacharya, Steven Kotsopoulos, Jeff Olson, Valérie Taly, Andrew D. Griffiths, Darren R. Link and Jonathan W. Larson
Lab Chip, 2011, 11, 2167-2174
DOI: 10.1039/C1LC20126C
Micromolding of solvent resistant microfluidic devices
Theodorus J. A. Renckens, Dainius Janeliunas, Hilbert van Vliet, Jan H. van Esch, Guido Mul and Michiel T. Kreutzer
Lab Chip, 2011, 11, 2035-2038
DOI: 10.1039/C0LC00550A
Pinched flow coupled shear-modulated inertial microfluidics for high-throughput rare blood cell separation
Ali Asgar S. Bhagat, Han Wei Hou, Leon D. Li, Chwee Teck Lim and Jongyoon Han
Lab Chip, 2011, 11, 1870-1878
DOI: 10.1039/C0LC00633E
Systematic investigation of droplet generation at T-junctions
Thomas Schneider, Daniel R. Burnham, Jaylen VanOrden and Daniel T. Chiu
Lab Chip, 2011, 11, 2055-2059
DOI: 10.1039/C1LC20259F
Stand-alone self-powered integrated microfluidic blood analysis system (SIMBAS)
Ivan K. Dimov, Lourdes Basabe-Desmonts, Jose L. Garcia-Cordero, Benjamin M. Ross, Antonio J. Ricco and Luke P. Lee
Lab Chip, 2011, 11, 845-850
DOI: 10.1039/C0LC00403K
Mixing enhancement for high viscous fluids in a microfluidic chamber
Shasha Wang, Xiaoyang Huang and Chun Yang
Lab Chip, 2011, 11, 2081-2087
DOI: 10.1039/C0LC00695E
Miniature magnetic resonance system for point-of-care diagnostics
David Issadore, Changwook Min, Monty Liong, Jaehoon Chung, Ralph Weissleder and Hakho Lee
Lab Chip, 2011, 11, 2282-2287
DOI: 10.1039/C1LC20177H
On-chip background noise reduction for cell-based assays in droplets
Pascaline Mary, Angela Chen, Irwin Chen, Adam R. Abate and David A. Weitz
Lab Chip, 2011, 11, 2066-2070
DOI: 10.1039/C1LC20159J
Why not take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Lab on a Chip? Then why not submit to us today or alternatively email us your suggestions.
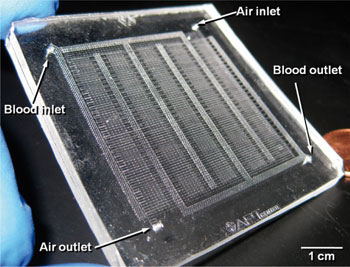
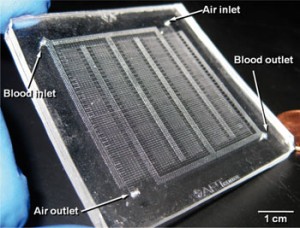 New artificial lung breathes like a real one
New artificial lung breathes like a real one
















 Another LOC article has been highlighted in
Another LOC article has been highlighted in  The image on the outside front cover depicts a microfluidic artificial photosynthesis platform created by
The image on the outside front cover depicts a microfluidic artificial photosynthesis platform created by  Highlighted on the inside front cover is another exciting article, demonstrating the 3D focussing of particles in a microfluidic channel using standing surface acoustic waves. The paper builds on previous work from
Highlighted on the inside front cover is another exciting article, demonstrating the 3D focussing of particles in a microfluidic channel using standing surface acoustic waves. The paper builds on previous work from