Pedro Lozano in a Professor of Biochemistry and Molecular Biology at the University of Murcia, Spain. His current research interests are related to enzyme technology with particular focus on the use of enzymes in ionic liquids and supercritical fluids. Pedro took time out from his work to speak to Green Chemistry…
Who or what initially inspired you to become a chemist?
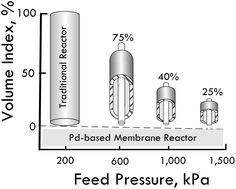
My interest in experimental sciences began during my training at High School, which awoke my passion to know and understand all the phenomena of Nature. The choice to be a chemist was really accidental, because belonging to a family with few economic resources I had to study at the public University nearest my home, the University of Murcia. In 1979, Chemistry was the only experimental sciences degree offered this university. However, during the last two years of training in Chemistry, I chose the specialty of Biochemistry, allowing me to discover not only that the essence of life is chemistry, but also the enormous potential of applying biological systems to develop chemical processes of industrial interest in the context of Biotechnology. In fact, my PhD dealt with a cross-flow membrane reactor with immobilized pectinases to continuously produce clarified fruit juices.
What has been the motivation behind your recent research?
During the 90’s, my research was focused on the application of proteases to peptide synthesis in non-conventional media, mainly organic solvents and supercritical fluids. I came across supercritical fluids during my postdoctoral training at the Centre de Bioengenierie Gilbert Durand at Toulouse. I realized that there was a whole world to discover using biocatalytic systems in combination with these new solvents. Then by chance, in 1999 I met Dr. Michel Vaultier who was visiting the University of Murcia, and it was he who introduced me to ionic liquids. Also, the paper of Prof Joan F. Brennecke, (Nature, 1999, 399, 28-29) was a source of inspiration. Since that time, the combination of the catalytic excellence of enzymes with the unique characteristics of both supercritical fluids and ionic liquids has been a constant in my research activities. The idea of being able to perform clean and continuous catalytic processes under non-aqueous conditions using the inherent advantages of enzymes for processes of industrial interest is the final aim. At this moment, both dynamic kinetic resolutions and the enzymatic synthesis of biodiesel are the two processes under study, because of their possible application to important strategic sectors of the pharmaceutical and biofuels industries, respectively. However, the small size of our laboratory, the lack of human and material resources and an excessive teaching load are clear limitations to any research development.
What do you see as the main challenges facing research in this area?
Today, the big challenge, not only in this field, is to be able to do research in Spain in the deep economic crisis.
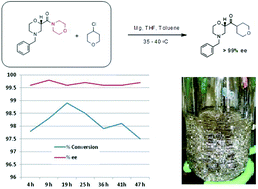
Outside this context, to provide a sufficiently high level of activity and stability to the enzymes to carry out synthetic processes in an overall reaction and separation approach is an important challenge. In the case of chemoenzymatic processes, it is important to have an active and selective chemical catalyst for the racemisation step, as it must also preserve (or at least, not destroy) the catalytic properties of enzymes for the kinetic resolution step. For instance, we have developed appropriate chemoenzymatic systems for the dynamic kinetic resolution of a sec-arylalcohol, but there are other sec-compounds like sec-amines, sec-thiols, etc. (in aryl and alkyl compounds), but there are many candidate reactions waiting to be studied. In the case of the enzymatic synthesis of biodiesel, our original contribution concerned the use of ILs with a large alkyl-side chain in their cations. This provides efficient monophasic reaction systems for enzyme catalysis, and opened the door to their industrial application. The full transformation of vegetable oil into biofuel molecules, without the undesirable production of glycerol is a very interesting challenge for any multicatalytic system.
Where do you see the field of Green Chemistry being in 5 or 10 years time?
The main overall challenge for the 21st century is to ensure truly sustainable development, as it is defined by the Brundtland Report, “development that meets the needs of the present without compromising the ability of future generations to meet their own need”, where the implementation of the twelve Principles of Green Chemistry, masterfully defined by Prof. Paul T. Anastas, is a good roadmap to follow. In the next 10 years, the interest of the society in green chemistry will be at its highest level, because of the profound changes that it should be occur in the chemical industry sector. Green Chemistry research should make efforts to combine selective catalysts with clean reaction media, by using sustainable approaches for product recovery and to enable the recycling/reuse of these reaction media. To transfer the exquisite efficiency shown by enzymes in nature to chemical processes may constitute the most powerful toolbox for developing a clean and sustainable chemical industry in the near future.
And finally…
If you could not be a scientist, but could be anything else, what would you be?
Really, research is vocational, and provides scientists with the enormous pleasure of having a job they enjoy. In my case, I am very lucky with my job. Furthermore, I like to work and I enjoy working…. As an undergraduate, I had many jobs during the summers to earn money to finance my studies. So, if I had not been a scientist, I do not know what other job had I would have chosen, but I am sure that it would be a job of service to society, which I would enjoy.
Take a look a couple of Pedro’s recent articles in Green Chemistry – free to access until the 22nd May 2012:
Stabilizing immobilized cellulase by ionic liquids for saccharification of cellulose solutions in 1-butyl-3-methylimidazolium chloride, Pedro Lozano, Berenice Bernal, Juana M. Bernal, Mathieu Pucheault and Michel Vaultier, Green Chem., 2011, 13, 1406-1410
An efficient activity ionic liquid-enzyme system for biodiesel production, Teresa De Diego, Arturo Manjón, Pedro Lozano, Michel Vaultier and José L. Iborra, Green Chem., 2011, 13, 444-451
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.
Comments Off on Meet our Authors: Pedro Lozano