On the 16th–18th June 2014, the Cluster of Excellence “Tailor-Made Fuels from Biomass (TMFB)” at RWTH Aachen University will organize it’s 2nd International Conference on Biofuel production and combustion. After their International Workshops were turned into an open Conference for the first time in 2013, the Aachen scientists continue to transfer their interdisciplinary research approach to their International Conference which is organized in different sessions that reflect the research structure of the Cluster of Excellence:
The following topics will be addressed in separate sessions during the conference:
• Biomass Fractionation and Pre-treatment
• Enzymatic and Catalytic Biomass Processing
• Catalytic Synthesis and Conversion of Biomass-based Streams to Platform Molecules and Fuels
• (Bio-)refinery Process Optimization
• Injection, Ignition and Combustion of Biofuels
• Combustion Process and Exhaust Gas After treatment Optimization of Biofuels
Call for Papers: If you would like to contribute to this conference with a presentation of your work in one of the above fields, please send in a one-page summary of your topic – click here for full details on how to submit. The deadline for submission is the 31st January 2014.
Confirmed Invited Speakers
The conference sessions will complemented by key note lectures from experts of all addressed disciplines:
• Gabriele Centi, Professor of Industrial Chemistry, University of Messina, the Netherlands
• André Faaij, Professor for Energy System Analysis, University of Utrecht, the Netherlands
• Tiziano Faravelli, Professor for Chemical Reaction Engineering and Chemical Kinetics, Politecnico di Milano, Italy
• Kohsuke Honda, Professor for Biotechnology, Osaka University, Japan
• Luuk van der Wielen, Distinguished Professor for Biobased Economy, Delft University of Technology and President of the Executive Board of BE-Basic Foundation, the Netherlands
• Marcel Wubbolts, CTO, Royal DSM, Urmond, the Netherlands
Visit the conference website for more information.











 The front cover this month (pictured left) features work by Peter C. K. Lau and co-workers from Quebec, Canada. In their work they engineer sinapic acid decarboxylaseas an alternative to chemistry-based or thermal decarboxylation to produce canolol from canola meal.
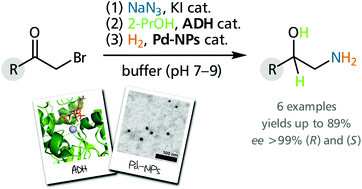
The front cover this month (pictured left) features work by Peter C. K. Lau and co-workers from Quebec, Canada. In their work they engineer sinapic acid decarboxylaseas an alternative to chemistry-based or thermal decarboxylation to produce canolol from canola meal. The inside front cover this month (pictured right) features work by Joerg Schrittwieser, Frank Hollmann and co-workers from Deltf, The Netherlands. In their work they show how the one-pot combination of alcohol dehydrogenase (ADH) and palladium nanoparticle (Pd-NP) catalysis provides access to aromatic 1,2-amino alcohols in high yields and excellent optical purities.
The inside front cover this month (pictured right) features work by Joerg Schrittwieser, Frank Hollmann and co-workers from Deltf, The Netherlands. In their work they show how the one-pot combination of alcohol dehydrogenase (ADH) and palladium nanoparticle (Pd-NP) catalysis provides access to aromatic 1,2-amino alcohols in high yields and excellent optical purities.



 The front cover this month (pictured left) features work by Wouter De Soute and co-workers from Ghent, Belgium. In their work, they show that shifting from batch to continuous pharmaceutical tablet manufacturing results in a significant reduction in natural resource extraction.
The front cover this month (pictured left) features work by Wouter De Soute and co-workers from Ghent, Belgium. In their work, they show that shifting from batch to continuous pharmaceutical tablet manufacturing results in a significant reduction in natural resource extraction.  The inside front cover this month (pictured right) features work by Davit Zargarian and co-workers from Quebec, Canada. In their work they reveal a new one-pot method for the efficient and atom-economical synthesis of POCOP-type pincer complexes of divalent nickel that serve as pre-catalysts for various catalytic transformations.
The inside front cover this month (pictured right) features work by Davit Zargarian and co-workers from Quebec, Canada. In their work they reveal a new one-pot method for the efficient and atom-economical synthesis of POCOP-type pincer complexes of divalent nickel that serve as pre-catalysts for various catalytic transformations.