Issue 10 of Green Chemistry is now available to read online.
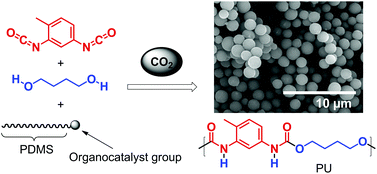
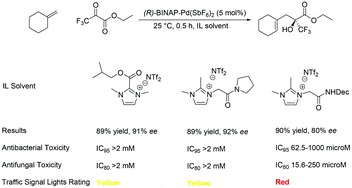
The front cover this month (pictured left) features work by Nicholas Gathergood and Stephen Connon and co-workers from the Czech Republic, Spain and Ireland. In their series of three back-to-back papers, they report how consideration of green chemistry metrics in tandem with performance studies can lead to prioritising safer ionic liquid solvents and imidazolium derived catalysts. Metrics determined include – antimicrobial toxicity, biodegradation, atom economy and length of catalyst synthesis. Performance of the imidazolium derived catalysts was greatly enhanced by incorporation of either esters or amides into the heterocyclic core, leading to low catalyst loadings. A Traffic Signal Light classification system was used to conveniently compare ionic liquid solvents and imidazolium derived catalysts metrics.
Read the three articles in full online:
Tandem ionic liquid antimicrobial toxicity and asymmetric catalysis study: carbonyl-ene reactions with trifluoropyruvate
Rohitkumar G. Gore, Thi-Kim-Thu Truong, Milan Pour, Lauren Myles, Stephen J. Connon and Nicholas Gathergood
Green Chem., 2013, 15, 2727-2739, DOI: 10.1039/C3GC40875B
A new generation of aprotic yet Brønsted acidic imidazolium salts: low toxicity, high recyclability and greatly improved activity
Lauren Myles, Rohitkumar G. Gore, Nicholas Gathergood and Stephen J. Connon
Green Chem., 2013, 15, 2740-2746, DOI: 10.1039/C3GC40975A
A new generation of aprotic yet Brønsted acidic imidazolium salts: effect of ester/amide groups in the C-2, C-4 and C-5 on antimicrobial toxicity and biodegradation
Rohitkumar G. Gore, Lauren Myles, Marcel Spulak, Ian Beadham, Teresa M. Garcia, Stephen J. Connon and Nicholas Gathergood
Green Chem., 2013, 15, 2747-2760, DOI: 10.1039/C3GC40992A
Work by the team has also been featured in a recent Feature Article in Chemistry World. Click here to read the Feature Article in full.

Read the full article:
Mechanical depolymerisation of acidulated cellulose: understanding the solubility of high molecular weight oligomers
Abhijit Shrotri, Lynette Kay Lambert, Akshat Tanksale and Jorge Beltramini
Green Chem., 2013, 15, 2761-2768, DOI: 10.1039/C3GC40945G
All of these articles are free to access for 6 weeks!
Keep up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.