Issue 4 of Green Chemistry is a part themed issue on ‘Elemental Recovery and Sustainability‘ focusing on how we can develop methods to ensure that elements are available for use by future generations through sustainable use and recovery.
The guest editors for this themed collection are James Clark (University of York, UK), Andrew Hunt (University of York, UK), Avtar Matharu (University of York, UK) and Alex King (Ames Labs, USA), read their editorial for free here.
 The outside front cover of the issue features the Critical Review “Bio-derived materials as a green route for precious & critical metal recovery and re-use” by Jennifer R. Dodson, Helen L. Parker, Andrea Muñoz García, Alexandra Hicken, Kaana Asemave, Thomas J. Farmer, He He, James H. Clark and Andrew J. Hunt. In this article they give an overview of research in critical and precious metal recovery using biosorption, application to real-life wastes and uses of the metal-loaded materials.
The outside front cover of the issue features the Critical Review “Bio-derived materials as a green route for precious & critical metal recovery and re-use” by Jennifer R. Dodson, Helen L. Parker, Andrea Muñoz García, Alexandra Hicken, Kaana Asemave, Thomas J. Farmer, He He, James H. Clark and Andrew J. Hunt. In this article they give an overview of research in critical and precious metal recovery using biosorption, application to real-life wastes and uses of the metal-loaded materials.
The inside front cover of the issue features the Paper “Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]” by David Duponta and Koen Binnemans. In this article they describe how a new recycling process was developed to recover rare earths from roasted NdFeB magnets using the thermomorphic and acidic properties of the ionic liquid [Hbet][Tf2N] to achieve a combined leaching/extraction system.
These two articles are free to access until 15th May and there are also a number of open access articles within the issue:















 The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates.
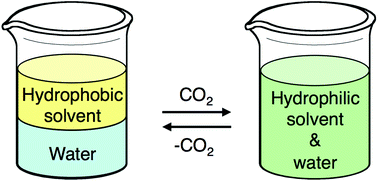
The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates. The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.
The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.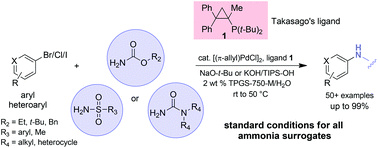
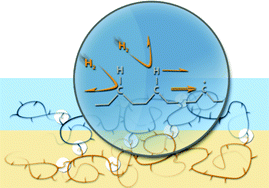
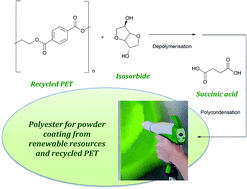





 The inside front cover this month (pictured right) features work by Jorge Beltramini and co-workers from Brisbane, Australia. In their work they utilize high resolution NMR to explain the depolymerisation mechanism of cellulose during acid treatment and milling.
The inside front cover this month (pictured right) features work by Jorge Beltramini and co-workers from Brisbane, Australia. In their work they utilize high resolution NMR to explain the depolymerisation mechanism of cellulose during acid treatment and milling.