Check out the following HOT articles, these have all been made free to access for a limited time:
Recycling of rare earths from NdFeB magnets using a combined leaching/extraction system based on the acidity and thermomorphism of the ionic liquid [Hbet][Tf2N]
David Dupont and Koen Binnemans
Green Chem., 2015,17, 2150-2163
DOI: 10.1039/C5GC00155B
Upgrading biogenic furans: blended C10–C12 platform chemicals via lyase-catalyzed carboligations and formation of novel C12 – choline chloride-based deep-eutectic-solvents 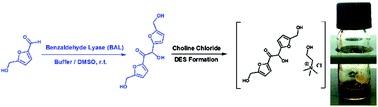
Joseph Donnelly, Christoph R. Müller, Lotte Wiermans, Christopher J. Chuck and Pablo Domínguez de María
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00342C
From simple organobromides or olefins to highly value-added bromohydrins: a versatile performance of dimethyl sulfoxide
Song Song, Xiaoqiang Huang, Yu-Feng Liang, Conghui Tang, Xinwei Lia and Ning Jiao
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00184F
Extraction and separation of neodymium and dysprosium from used NdFeB magnets: an application of ionic liquids in solvent extraction towards the recycling of magnets
Sofía Riaño and Koen Binnemans
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00230C, Paper
Greening the global phosphorus cycle: how green chemistry can help achieve planetary P sustainability
Paul J. A. Withers, James J. Elser, Julian Hilton, Hisao Ohtake, Willem J. Schipper and Kimo C. van Dijk
Green Chem., 2015,17, 2087-2099
DOI: 10.1039/C4GC02445A, Perspective
Stimuli-responsive/rheoreversible hydraulic fracturing fluids as a greener alternative to support geothermal and fossil energy production 
H. B. Jung, K. C. Carroll, S. Kabilan, D. J. Heldebrant, D. Hoyt, L. Zhong, T. Varga, S. Stephens, L. Adams, A. Bonneville, A. Kuprat and C. A. Fernandez
Green Chem., 2015, Advance Article
DOI: 10.1039/C4GC01917B, Paper
Fluorine gas for life science syntheses: green metrics to assess selective direct fluorination for the synthesis of 2-fluoromalonate esters
Antal Harsanyi and Graham Sandford
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00402K, Paper
Layered MoS2 nanoparticles on TiO2 nanotubes by a photocatalytic strategy for use as high-performance electrocatalysts in hydrogen evolution reactions
Chenhui Meng, Zhaoyue Liu, Tierui Zhang and Jin Zhai
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00272A, Communication
Ionic liquid-stabilized nanoparticles as catalysts for the conversion of biomass
K. L. Luska, P. Migowski and W. Leitner
Green Chem., 2015, Advance Article
DOI: 10.1039/C5GC00231A, Critical Review
















 The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates.
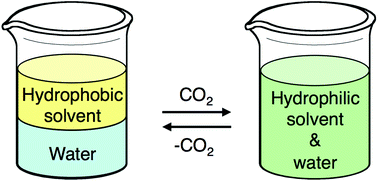
The outside front cover this month (pictured left) features work by Javier Pérez-Ramírez and co-workers from Zurich, Switzerland. In their work they report how the Lewis-acid catalysed isomerisation of bio-oil derived glyocal over tin-based zeolites efficiently and sustainably produces glycolic acid and alykyl glycolates. The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.
The inside front cover this month (pictured right) features work by Philip Jessop and co-workers from Ontario, Canada. In their work they focus on switchable-hydrophilicity solvents (SHS), which can switch reversibly between one form that is miscible with water and another that forms a biphasic mixture with water. They report new examples and compare them in terms of safety and environmental impact.