 The latest issue of Green Chemistry is now available online.
The latest issue of Green Chemistry is now available online.
The front cover of this issue features work by Rodrigo Teixeira from the University of Alabama in Huntsville, USA who reports the energy efficient extraction of fuel and chemical feedstocks from algae. The deconstruction of algae cell walls and release of the cells contents was achieved by dissolution and hydrolysis of wet algae biomass in ionic liquids. This process does not require acids, bases or any other catalysts, and can be completed in less than 50 mins (regardless of the algae species) at 100-140 °C and atmospheric pressure.
Energy-efficient extraction of fuel and chemical feedstocks from algae, Rodrigo E. Teixeira, Green Chem., 2012, 14, 419-427
 The inside front cover highlights work by Richard Daniellou, Daniel Plusquellec and colleagues from the National School of Chemistry of Rennes and the European University of Brittany, France, who report aqueous solutions of facial amphiphilic carbohydrates as a sustainable media for organocatalyzed direct aldol reactions. Their system was applied to the direct aldol reaction of m-nitrobenzaldehyde with various cyclohexanones, and proceeded with high yields, shortened reaction times and improved diastereoselectivities.
The inside front cover highlights work by Richard Daniellou, Daniel Plusquellec and colleagues from the National School of Chemistry of Rennes and the European University of Brittany, France, who report aqueous solutions of facial amphiphilic carbohydrates as a sustainable media for organocatalyzed direct aldol reactions. Their system was applied to the direct aldol reaction of m-nitrobenzaldehyde with various cyclohexanones, and proceeded with high yields, shortened reaction times and improved diastereoselectivities.
Aqueous solutions of facial amphiphilic carbohydrates as sustainable media for organocatalyzed direct aldol reactions, Ana Bellomo, Richard Daniellou and Daniel Plusquellec, Green Chem., 2012, 14, 281-284
Read these articles for free until the 14th March 2012!
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts!











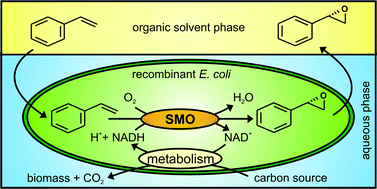
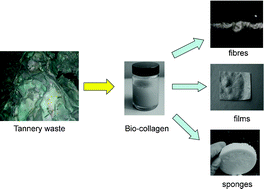
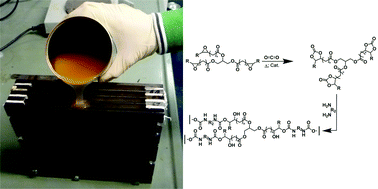
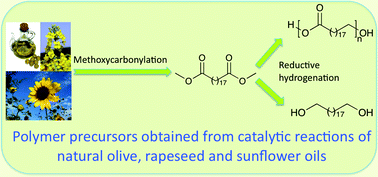

 Anett Schallmey and Bruno Bühler,
Anett Schallmey and Bruno Bühler, 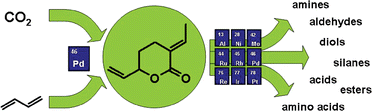 Use of carbon dioxide in chemical syntheses via a lactone intermediate
Use of carbon dioxide in chemical syntheses via a lactone intermediate