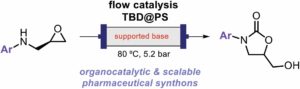
Assessing the environmental benefit of palladium-based single-atom heterogeneous catalysts for Sonogashira coupling
D. Faust Akl, D. Poier, S. C. D’Angelo, T. P. Araújo, V. Tulus, O. V. Safonova, S. Mitchell, R. Marti, G. Guillén-Gosálbez and J. Pérez-Ramírez
Green Chem., 2022, 24, 6879-6888. DOI: 10.1039/D2GC01853E
|
Green foundation |
|
|
- This study quantitatively assesses through life-cycle analysis (LCA) possible environmental benefits of replacing soluble palladium organometallic complexes with single-atom heterogeneous catalysts in cross-coupling reactions, exemplified for the Sonogashira reaction.
- Reusing the heterogeneous catalyst once, assuming its stability and full metal retention, can already deliver advantages in various impact categories over the conventional homogeneous systems, with the potential for orders-of-magnitude improvements. The LCA results provide criteria for implementing solid, reusable catalysts in sustainable organic transformations.
- In future work, the presented LCA framework may inform catalyst design and help streamline research efforts toward more sustainable catalytic materials.
|
|
|
High performance, but low cost and environmental impact? Integrated techno-economic and life cycle assessment of polyoxazolidinone as a novel high-performance polymer
M. Bachmann, A. Marxen, R. Schomäcker and A. Bardow
Green Chem., 2022, 24, 9143-9156. DOI:10.1039/D2GC02400D
|
Green foundation |
|
|
- This work assesses the economic and environmental potential of polyoxazolidinones (POX) as high-performance thermoplastics.
- A combined techno-economic and life-cycle assessment shows that POX reduce the carbon footprint of high-performance thermoplastics at competitive costs even for fossil-based production. Employing biomass could further reduce the carbon footprint but would introduce environmental trade-offs such increasing freshwater eutrophication.
- POX are identified as promising as high-performance thermoplastics, but the assumed material performance needs to be confirmed experimentally and environmental trade-offs considered in detail before large-scale implementation.
|
|
|
Early-stage impact assessment tool (ESTIMATe) for the life cycle assessment of CO2-based chemicals
H. Minten, B. D. Vandegehuchte, B. Jaumard, R. Meys, C. Reinert and A. Bardow
Green Chem., 2024, 26, 8728-8743. DOI:10.1039/D4GC00964A
|
Green foundation |
|
|
- This work introduces a software tool allowing non-experts to perform early-stage life-cycle assessment for CO2 conversion processes.
- The open-source Excel tool ESTIMATe is provided that automates and streamlines life-cycle assessment of carbon capture and utilization processes. LCA assumptions are automated and estimation tools are provided to fill data gaps. Thereby, ESTIMATe makes environment assessments accessible to non-experts even at early-stages of development.
- Deployment of the ESTIMATe tool will hopefully improve early-stage decision making and also help to refine the tool itself. Future developments are to expand the scope beyond CO2 conversion.
|
|
|
Introducing the use of a recyclable solid electrolyte for waste minimization in electrosynthesis: preparation of 2-arylbenzoxazoles under flow conditions
F. Ferlin, F. Valentini, F. Campana and L. Vaccaro
Green Chem., 2024, 26, 6625-6633. DOI:10.1039/D4GC00930D
|
Green foundation |
|
|
- The work introduces the use of solid electrolyte into organic electrosynthesis, and it proves that with this approach is possible to significantly reduce the waste associated to the use of stochiometric classic homogeneous electrolyte generally containing halides
- Calculation of the green metrics (E-factors, RME, MRP) for the newly defined procedure and several literature examples, allow to quantify the specific achievement. E-factor has been reduced of ca. 82-99%. Mass of the electrolyte generally constitutes 25–68% of the entire E-kernel and in our case, we could obtain a very low value of 0.12%.
- Future research will be dedicated to expanding the utilization of solid electrolyte in different electroassisted processes using with safe recoverable reaction media.
|
|
|
Valorisation of phenols to coumarins through one-pot palladium-catalysed double C–H functionalizations
G. Brufani, F. Valentini, F. Sabatelli, B. Di Erasmo, A. M. Afanasenko, C.-J. Li and L. Vaccaro
Green Chem., 2022, 24, 9094-9100. DOI:10.1039/D2GC03579K
|
Green foundation |
|
|
- The use of a novel synthetic strategy based on the Pd/C catalysed C−H functionalization of substituted phenols has allowed the direct synthesis of prenylated coumarins. The multistep protocols for the synthesis of osthole-like derivatives, which frequently use toxic reagents and non-recyclable catalysts, could be replaced using our one-pot procedure, which has proven to be efficient in the synthesis of biologically active products.
- Our newly procedures for the synthesis of osthole derivates showed significant improvement in terms of atom economy, from 32% to 81%. For direct comarin synthesis, the waste was reduced up to 56% and the efficient recovery and reuse of heterogeneous catalytic system has allowed a TON value of 41 (over 5 consecutive runs) which is greater than the value obtained for analogous homogeneous systems (6.2–32.3).
- Further work on the kinetics and the mechanism would aid in future research particularly for other derivatives
|
|
|
Aerobic waste-minimized Pd-catalysed C–H alkenylation in GVL using a tube-in-tube heterogeneous flow reactor
F. Ferlin, I. Anastasiou, L. Carpisassi and L. Vaccaro.
Green Chem., 2021, 23, 6576-6582. DOI:10.1039/D1GC01870A
|
Green foundation |
|
|
- We utilize an efficient flow reactor system for the Fujiwara–Moritani C–H alkenylation reaction of biomass-derived γ-valerolactone. The protocol features very limited metal leaching, high stability of the catalyst, and applicability to a range of substituted acetanilides and N-methoxybenzamides and others.
- By using the flow reactor system, the external oxidant could be minimised, which also reduced leaching of the palladium catalyst (from ca. 4 ppm to 0.2–0.02 ppm). The use of biomass derived GVL as the reaction medium also reduced metal leaching by almost an order of magnitude to the next best solvent. By comparing to protocols in the literature, the E-factor value of our newly defined protocol is 80->99% lower and the reaction mass efficiency and materials recovery parameter are noticeably improved.
- When scaling up, an efficient recovery process for the leached palladium would be critical for a sustainable system.
|
|
|
Non-noble metal heterogeneous catalysts for hydrogen-driven deoxydehydration of vicinal diol compounds
J. Gan, Y. Nakagawa, M. Yabushita and K. Tomishige.
Green Chem., 2024, 26, 8267-8281. DOI:10.1039/D4GC02006E
|
Green foundation |
|
|
- From the viewpoint of carbon neutrality and carbon recycling, the synthesis of biomass to valuable chemicals using greener catalysts is increasingly important. The present work shows the development of non-noble metal catalysts for deoxydehydration (DODH).
- Rather than use noble metal catalysts like Re or Au, we show the development and activity of a range of non-noble metal catalysts for the DODH reaction of 1,4-anhydroerythritol, a typical biomass-derived platform molecule, and demonstrate comparable yields of the target product. The new catalyst could be reused after calcination without loss of activity.
- An environmental impact assessment of the catalyst preparation and the final process could help guide the next steps.
|
|
|
Accessing secondary amine containing fine chemicals and polymers with an earth-abundant hydroaminoalkylation catalyst
M. Manßen, S. S. Scott, D. Deng, C. H. M. Zheng and L. L. Schafer.
Green Chem., 2023, 25, 2629-2639. DOI:10.1039/D3GC00011G
|
Green foundation |
|
|
- We present a titanium-catalysed hydroaminoalkylation process, as a greener alternative to the industrially accepted hydroaminomethylation transformation, which relies on rhodium hydroformylation catalysts.
- Our Ti(NMe2)4/ligand system showed activity for an increased diversity of substrates, excellent regioselectivity, simple catalyst design, and quantifiable improvement on standard environmental assessment metrics. Compared to the best-in-class tantalum hydroaminoalkylation catalyst, our catalyst based on Ti(NMe2)4/ligand was shown by LCA to be less impactful in five out of nine categories for amine terminated polypropylene synthesis.
- This Ti(NMe2)4/ligand system could also be used for challenging postpolymerisation of macromolecular substrates, where this catalyst and process would offer a greener alternative.
|
|
|
Ultrasonic-assisted oxidation of cellulose to oxalic acid over gold nanoparticles supported on iron-oxide
P. N. Amaniampong, Q. T. Trinh, T. Bahry, J. Zhang and F. Jérôme.
Green Chem., 2022, 24, 4800-4811. DOI:10.1039/D2GC00433J
|
Green foundation |
|
|
- The global market for oxalic acid was around 1340 thousand tons in 2022. Here we present a greener alternative to the harsh conditions regularly required in industry to overcome the recalcitrance of cellulose in chemical processing.
- We demonstrate that low frequency ultrasound induces the fragmentation of cellulose particles to facilitate the otherwise highly challenging, base-free oxidation of cellulose to oxalic acid. We show that ultrasonic conditions lead to partial fragmentation of cellulose particles, making it more reactive with the catalyst.
- Full elucidation and greater understanding of the role of ultrasonic conditions on the reaction mechanism is required.
|
|
|
Development of a solvent sustainability guide for the paints and coatings industry
L. Pilon, D. Day, H. Maslen, O. P. J. Stevens, N. Carslaw, D. R. Shaw and H. F. Sneddon.
Green Chem., 2024, Advance Article. DOI:10.1039/D4GC01962H
|
Green foundation |
|
|
- The paints and coatings industry has increasingly been moving towards lower emissions and the nature of the solvents considered in future is anticipated to come under increasing scrutiny. A solvent sustainability guide is offered for the paints and coatings industry, considering solvents likely of interest in this sector, and considering criteria relevant to these applications.
- A range of solvents relevant to this sector were compared. While instances where like-for-like drop-in replacements can be identified are expected to be few, the guide allows ready identification of a range of greener or more sustainable solvents as possible start points for further formulation research.
- New solvent data continues to be collected, and regulations evolve, therefore, it is essential to only to use this guide in conjunction with reliable sources to obtain the most current information.
|
|
|
Selectivity switch by phase switch – the key to a high-yield furfural process
L. Ricciardi, W. Verboom, J.-P. Lange and J .Huskens
Green Chem., 2021, 23, 8079-8088. DOI:10.1039/D1GC01752G
|
Green foundation |
|
|
- Furfural is a versatile intermediate for biofuels and bio-based chemicals. We present a greener alternative to the industrial process that converts xylose-rich hydrolysate to furfural with higher yield.
- High xylose-to-furfural yield at and above approx. 90 mol%, becomes feasible with a variety of solvent combinations and phenylboronic acid concentrations. A conceptual process design for scaled-up furfural production is presented, where the produced furfural could be recovered from the system with modest losses of the solvents in the waste streams, and thus minimizing waste and maximizing recyclability.
- Additional research is needed to (1) finetune the selection of solvent and boronic acid to further minimize losses and toxicity/environmental risks, (2) validate the process concept and (3) deliver the information needed for designing the major pieces of equipment.
|
|
|