Here are the latest HOT papers published in Green Chemistry, as recommended by the referees:
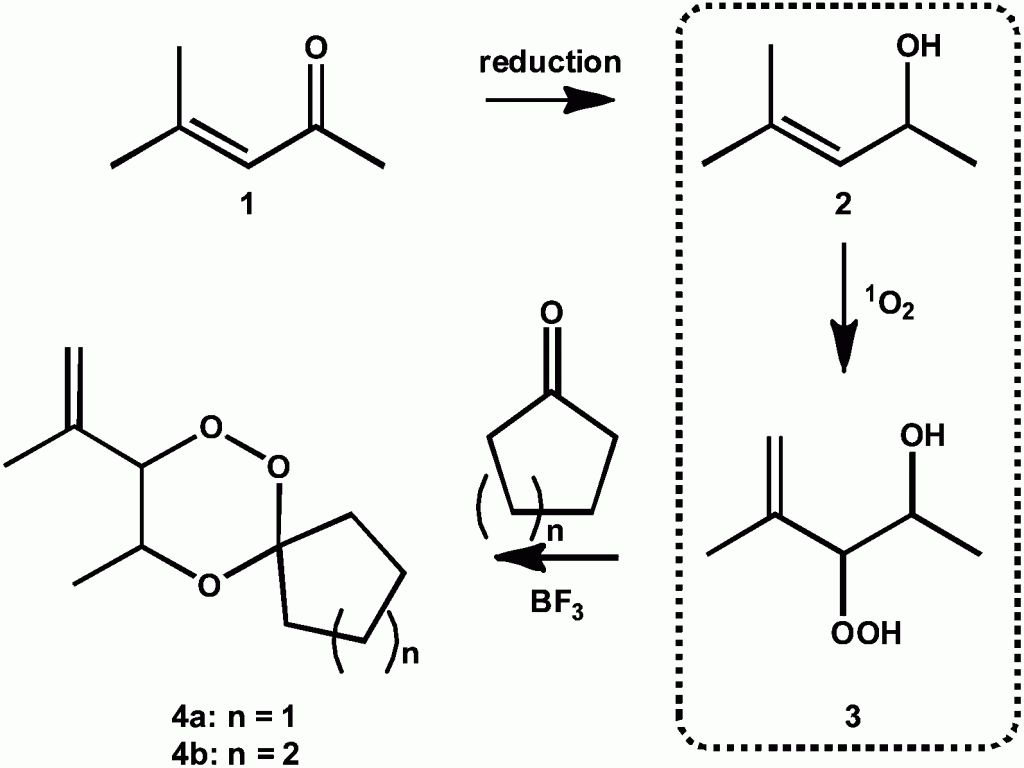
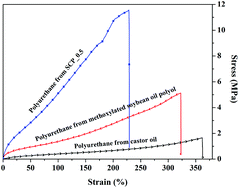
Soy-castor oil based polyols prepared using a solvent-free and catalyst-free method and polyurethanes therefrom
Chaoqun Zhang, Ying Xia, Ruqi Chen, Seungmoo Huh, Patrick A. Johnston and Michael R. Kessler
Green Chem., 2013,15, 1477-1484, DOI: 10.1039/C3GC40531A
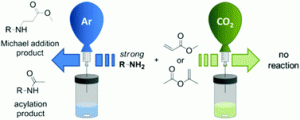
Carbon dioxide as a reversible amine-protecting agent in selective Michael additions and acylations
Annelies Peeters, Rob Ameloot and Dirk E. De Vos
Green Chem., 2013,15, 1550-1557, DOI: 10.1039/C3GC40568K
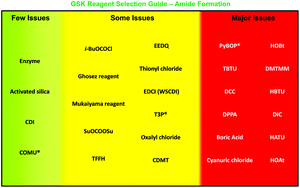
Development of GSK’s reagent guides – embedding sustainability into reagent selection
Joseph P. Adams, Catherine M. Alder, Ian Andrews, Ann M. Bullion, Matthew Campbell-Crawford, Michael G. Darcy, John D. Hayler, Richard K. Henderson, Catriona A. Oare, Israil Pendrak, Anikó M. Redman, Leanna E. Shuster, Helen F. Sneddon and Matthew D. Walker
Green Chem., 2013,15, 1542-1549, DOI: 10.1039/C3GC40225H
All the papers listed above are free to access for the next 4 weeks!












 Although some of the waste ash produced from the combustion of biomass is currently used in construction, most of it ends up in landfill. Therefore, extracting alkali silicates, which can be used in cement, detergents, catalysts and catalyst supports, is one way of reusing the potentially huge quantities of ash due to be produced in the future.
Although some of the waste ash produced from the combustion of biomass is currently used in construction, most of it ends up in landfill. Therefore, extracting alkali silicates, which can be used in cement, detergents, catalysts and catalyst supports, is one way of reusing the potentially huge quantities of ash due to be produced in the future. Recycling old magnets, so that rare-earth metals can be re-used, could help to solve
Recycling old magnets, so that rare-earth metals can be re-used, could help to solve 
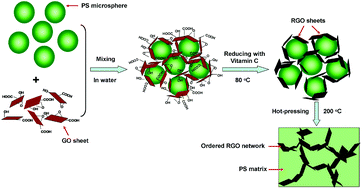
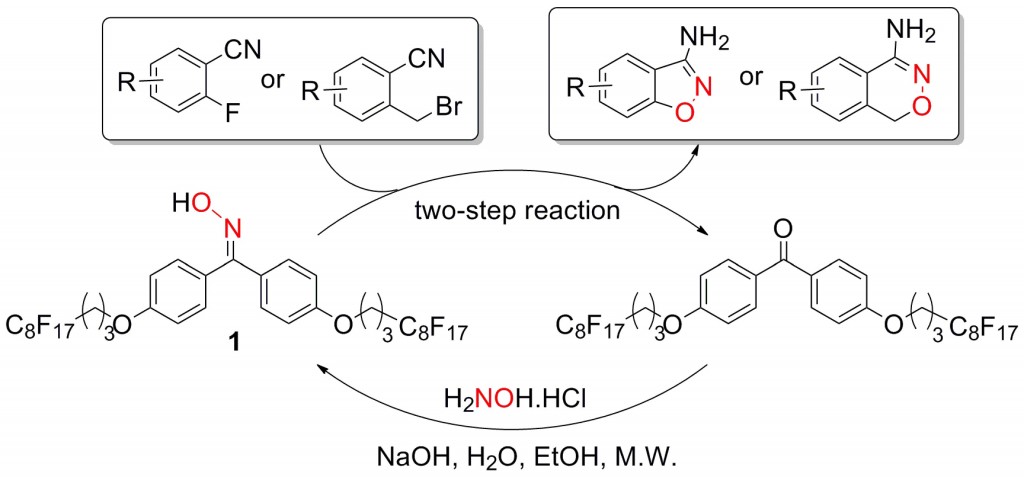
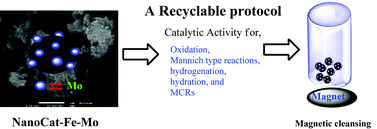
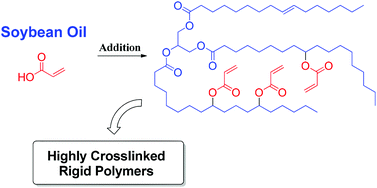

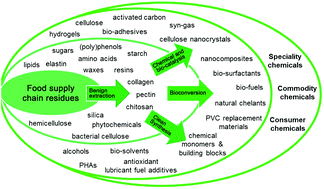
 The front cover of this month’s issue highlights the work of
The front cover of this month’s issue highlights the work of  The inside front cover features the work by
The inside front cover features the work by