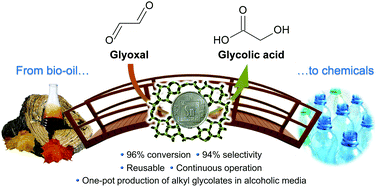
The European Commission has established a target for EU member states to obtain 20% of their energy from renewable sources by the year 2020. Although production of electricity from solar energy and hydropower are crucial technologies in achieving this goal, liquid hydrocarbon fuels are seemingly irreplaceable in certain heavy transportation sectors (specifically sea freight and aviation).
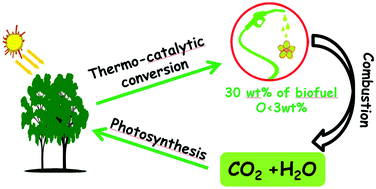
Scientific advances are now being made in the use of non-food crops to produce liquid hydrocarbon fuels, complementing the established oxygenated biofuels made of ethanol and bio-diesel. The latest research demonstrates that pine wood can be successfully converted into a mixture of liquid hydrocarbons. The resulting fuel has a similar calorific value to diesel, and contains less than 5% oxygen.
The necessary catalyst is made through a simple wet impregnation technique to give copper and ruthenium supported on phosphotungstic acid, which is then calcined. The transformation of the lignocellulosic biomass is conducted under hydrogen at an elevated temperature to produce the liquid fuel (30 wt%), which separates from an aqueous phase and any residual solid. The organic liquid was found to contain a number of mostly cyclic aliphatic hydrocarbons and also aromatic compounds, and thus is an attractive option as a next generation biofuel.
Direct thermocatalytic transformation of pine wood into low oxygenated biofuel
Walid Al Maksoud, Cherif Larabi, Anthony Garron, Kai C. Szeto, Jean J. Walter and Catherine C. Santini
Green Chem., 2014, Advance Article, DOI: 10.1039/C3GC42596G













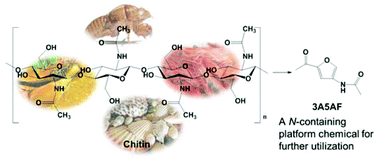
 Shrimp shells that would otherwise be thrown away by the seafood industry have been turned into
Shrimp shells that would otherwise be thrown away by the seafood industry have been turned into