| On the ethenolysis of natural rubber and squalene. Plenio and Wolf report the utilisation of natural rubber and squalene to obtain a set of terminal olefins. The main focus for producing olefins from renewable resources has been on plant oils. Despite the fact that the annual production (2007) of natural rubber was 9.7 × 106 tons, this polyolefin has not been widely used. However, due to the high degree of stereoregularity (in terms of double bond geometry and orientation) of natural rubber and squalene, Plenio was able to obtain controlled polymer degradation which resulted in the synthesis of sveral small oligoisoprenes. Future work is going to be directed scaling up this reaction and ultizing the products for the synthesis of flavours, odorants, etc. (Green Chem., 2011, DOI: 10.1039/c1gc15265c) | 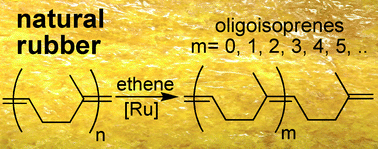 |
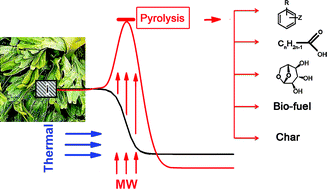 |
Microwave-mediated pyrolysis of macro-algae. Macro-algae is an abundant but generally underutilised resource – currently less that 1% is used. Its integration into a biofrinery is challenging as it contains increased levels of halogenated compounds, alkali earth and heavy metals which restrict its use in direct combustion. In this work, Clark and co-workers report the effective pyrolysis of macro-algae using a microwave. Pyrolysis is an established technique for deconstructing biomass using heat under an inert atmosphere to obtain high-value chemicals and platform molecules. In this study chemical rich bio-oils were obtained from macro-algae at temperatures lower than those used for conventional biomass and by using microwave irradiation they obtained a higher yield (21%) than that obtained with conventional heating (14%). (Green Chem., 2011, DOI: 10.1039/c1gc15560a) |
Archive for the ‘Hot Article’ Category
Utilizing natural resources – ethenolysis of natural rubber and pyrolysis of macro-algae
Critical reviews on selective oxidation of glycerol and recycling homogeneous catalysts using mebrane separation.
| Selective catalytic oxidation of glycerol – perspectives for high value chemicals. The increasing worldwide production of biodiesel has led to an excess supply of crude glycerol, prompting researchers to investigate its valorisation. Thanks to its three hydroxyl groups, glycerol is a potential starting material for several high-value fine chemicals. Various metals can catalyse glycerol oxidation. However, the selectively of these reactions is dependent on the active phase, metal particle size, pore size of the support and pH of the reaction medium. In this review, Dumeignil and co-workers look at the recent developments in new catalysts and spotlight the role of reaction conditions. (Green Chem., 2011, DOI: 10.1039/c1gc15320j) | 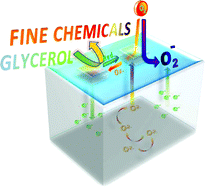 |
 |
Recent advances in recycling of homogeneous catalysts using membrane separation. Membrane filtration is now an attractive approach for the recycling of soluble catalysts. Nanofiltration has shown great potential as a method for process intensification in organo-, anzyme, and homogeneous catalysis. Vogt and co-workers discuss selected, recent advances in catalyst recovery by membrane filtration in this review and look at implications for future development. (Green Chem., 2011, DOI: 10.1039/c1gc15264e) |
Controlled lactide polymerisation in supercritical carbon dioxide with organo-catalyst
Researchers from Australia and the UK have developed a truly ‘green’ process for the synthesis of polylactic acid in the absence of toxic solvents and catalysts.
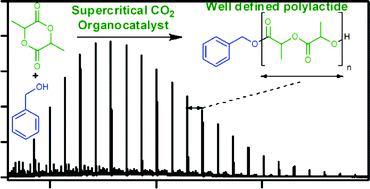
To read more, click the link below! FREE until 27 July.
Controlled polymerisation of lactide using an organo-catalyst in supercritical carbon dioxide, Idriss Blakey, Anguang Yu, Steven M. Howdle, Andrew K. Whittaker and Kristofer J. Thurecht, Green Chem., 2011, DOI: 10.1039/C1GC15344G
If you liked this, you may also be interested in this article too!
Direct conversion of polyamides to ω-hydroxyalkanoic acid derivatives by using supercritical MeOH, Akio Kamimura, Kouji Kaiso, Shuzo Suzuki, Yusuke Oishi, Yuki Ohara, Tsunemi Sugimoto, Kohichi Kashiwagi and Makoto Yoshimoto, Green Chem., 2011, DOI: 10.1039/C1GC15172J
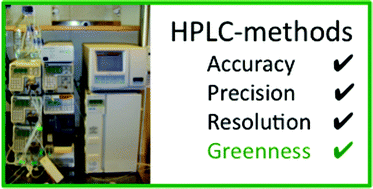
A free environmental assessment tool for liquid chromatography methods
Scientists from Sweden, Egypt, Denmark and India have collaborated to develop a computational tool to assess the greenness of high performance liquid chromatography (HPLC) methods.
The HPLC-EAT software can be downloaded free of charge at http://www.biotek.lu.se/hplc-eat/ and can also be combined with another free software eco-solvent tool to perform life cycle assessments of waste disposal options (such as distillation or incineration).
Click the link below to find out more! Read the full text for free until 22 July
Energy & environment: Free environmental assessment for liquid chromatography solvents, Y Gaber, U Tornvall, M A Kumar, M A Amin and R Hatti-Kaul, Green Chem., 2011, DOI: 10.1039/c0gc00667j
You may also be interested in the following article too!
Expanding GSK’s solvent selection guide – embedding sustainability into solvent selection starting at medicinal chemistry, Richard K. Henderson, Concepción Jiménez-González, David J. C. Constable, Sarah R. Alston, Graham G. A. Inglis, Gail Fisher, James Sherwood, Steve P. Binks and Alan D. Curzons, Green Chem., 2011, 13, 854-862 DOI: 10.1039/C0GC00918K
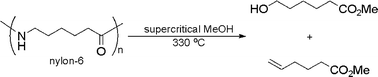
Recycling polyamides to give valuable chemicals
Polyamides have been decomposed to ω-hydroxyalkanoic acid derivatives – important intermediates in the chemical industry – using supercritical methanol. This is one of the first methods for economical upgrading of polymers through the depolymerisation process.
Akio Kamimura and colleagues from Yamaguchi University and UIbe Industries Ltd., Japan, have developed a method to convert polyamides to valuable chemicals. Currently, recycling of plastics commonly involves converting the polymers back to their monomers. However, these methods are not always economically viable because they are often more expensive than other ways of treating waste plastics. By converting polymers to valuable chemicals, this would make polymer recycling a more attractive route and solve some of the economic problems.
In this study, by using supercritical methanol, Kamimura converted nylon-6 selectively to two valuable compounds – methyl 6-hydroxycapronate and methyl 5-hexenoate. Given that the average price for these ω-hydroxyalkanoic acid derivatives is about 6-7 times higher (per kg, based on 2008 figures) than the monomer (caprolactam), Kamimura believes that this method will open up new avenues in the development of plastics recycling.
To read more, please click the link below! Full text Free until 20th July.
Direct conversion of polyamides to ω-hydroxyalkanoic acid derivatives by using supercritical MeOH, Akio Kamimura, Kouji Kaiso, Shuzo Suzuki, Yusuke Oishi, Yuki Ohara, Tsunemi Sugimoto, Kohichi Kashiwagi and Makoto Yoshimoto, Green Chem., 2011, DOI: 10.1039/C1GC15172J
Domino reaction to make heterocyclic spiro compounds
A new method has been developed to synthesis spiro-substituted benzo[b]furo[3,4-e][1,4]diazepene derivatives in one step, forming two spiro rings and five σ bonds in the process.
This chemo- and regioselective [4+2+1] domino cyclization reaction was discovered by scientists from Xuzhou Normal University, China and Texas Tech University, USA. The synthesis of heterocyclic compounds with quaternary carbon centres is important as these compounds are useful intermediates in organic synthesis and are often biologically active.
The procedure developed here is performed in water with microwave irradiation and uses easily available and cheap starting materials. This one-pot reaction can be performed in 10-18 minutes with reduced waste production and without the use of any strong acids or metal promoters. In addition, recrystallization and chromatography purification procedures can be avoided as the pure products can be obtained by simply washing the crude product with 95% ethanol.
The authors feel this will serve as a nice addition to Group-Assistant Purification chemistry.
Click the link below to read more! Full text free until 18th July.
[4+2+1] Domino cyclization in water for chemo- and regioselective synthesis of spiro-substituted benzo[b]furo[3,4-e][1,4]diazepine derivatives, Chuang Cheng, Bo Jiang, Shu-Jiang Tu and Guigen Li, Green Chem., 2011, DOI: 10.1039/C1GC15183E
“In tube” NMR procedure for analysis of Chiral alcohols and amines
An efficient method for the NMR analysis of chiral alcohols and amines has been developed with an improved detection limit. Valentine Ananikov and Nikolay Orlov, Zelinsky Institute of Organic Chemistry, Russia, prepared samples for analysis directly in the NMR tubes; this has big advantages over existing methods to analyse these compounds.
NMR analysis of chiral compounds normally involves derivatisation with a suitable chiral agent to allow you to tell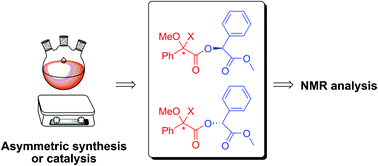
However, Ananikov derivatised samples within the NMR tube, avoiding waste generation and providing samples for analysis in less than 5 min (including preparation time). All analysis could be performed on a routine NMR instrument, and could give accurate measurements for samples less than 0.01 mg. This underlines the environmentally benign and superior nature of the procedure developed by this group.
To read more, please see:
NMR analysis of chiral alcohols and amines: development of an environmentally benign “in tube” procedure with high efficiency and improved detection limit, Nikolay V. Orlov and Valentine P. Ananikov, Green Chem., 2011, DOI: 10.1039/C1GC15191F
POM-based ionic hybrids for efficient heterogeneous epoxidation
A polyoxometalate (POM)-ionic liquid hybrid has been shown to be an efficient hereogeneous catalyst for the epoxidation of alkenes with hydrogen peroxide.
Scientists from China led by Jun Wang from Nanjing University of Technology, synthesied a hybrid material by anion-exchanging a amino-functionalised ionic liquid cation with Keggin POM-anions. These hybrid nanospheres were then applied to the epoxidation of alkenes with hydrogen peroxide. Previous methods using POM catalysts in this reaction have often suffered from slow reaction rates, complex catalyst preparation 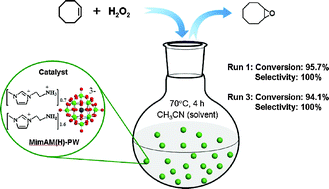
However, the POM-based ionic hybrid reported by Wang and co-workers, as well as being simple to produce, is an efficient heterogeneous catalyst exhibiting high conversion and selectivity. In addition it can by recycled several times (after simple filtration) without any observable loss in conversion or selectivity. Wang hopes that this new strategy may be extended to more versatile POM-based ionic hybrid catalysts for hydrogen peroxide, heterogeneous-based reactions.
To find out more, please see the full article, free until June 30th:
Polyoxometalate-based amino-functionalized ionic solid catalysts lead to highly efficient heterogeneous epoxidation of alkenes with H2O2, Yan Leng, Jun Wang, Dunru Zhu, Mingjue Zhang, Pingping Zhao, Zhouyang Long and Jun Huang, Green Chem., 2011, DOI: 10.1039/C1GC15302A
Bimetallic catalyst for hydrocarbon fuels, and styrene oxidation by hydrogen peroxide in ionic liquids
Production of liquid hydrocarbon fuels by catalytic conversion of biomass-derived levulinic acid. Dumesic and co-workers report the use of a highly active bimetallic catalyst for the conversion of levuilinic acid to γ-valerolactone (GVL). The RuRe/C catalyst used in this work is significantly more active that the traditional Ru/C, and shows stable activity and stereoselectivity in the presence of sulfuric acid. A model techno-economical analysis has shown that this new calaytic process can be integrated with the catalyic conversion of GVL to butene, followed by alkene oligomerization, and could provide a cost effective route for converting biomass into liquid hydrocarbon fuels. (Green Chem., 2011, DOI:10.1039/c1gc15047b)
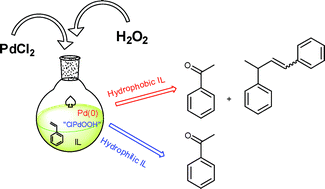
Styrene oxidation by hydrogen peroxide in ionic liquids: the role of the solvent on the competition between two Pd-catalyzed processes, oxidation and dimerization. In this work, Chiappe and her team investigated the role of solvent in the Wacker oxidation of styrene, in ionic liquids (ILs). They found that the nature of the IL has a strong influence on the outcome of the reaction. In the hydrophillic ILs, a homogeneous solution is formed and gives the expected product with high selectivity, and only a small amount of by-products. However, in hydrophobic ILs, the aqueous solution of hydrogen peroxide is immiscible and so forms a biphasic system. In this case side reactions occur, and the palladium catalyst is reduced from Pd(II) to Pd(0) and reoxidation back to Pd(II) is disfavoured. (Green Chem., 2011, DOI:10.1039/c0gc00945h)
Read these articles for free until 24th June
Using sunlight to drive electrochemical oxidations
Cheap and readily available photovoltaic cells have been used to conduct electrochemical oxidation reactions by Kevin D Moeller and co-workers from the Washington University in St Louis.
Oxidation reactions are among the most powerful tools available to synthetic chemists because they increase the level of functionality in a molecule. This study demonstrates that the energy efficiency of sunlight-driven reactions can be combined with the versatility of electrochemistry to create new, sustainable methods for conducting oxidation reactions.
Interested in knowing more? Read the full article here.
Connecting the dots: using sunlight to drive electrochemical oxidations
Laura A. Anderson, Alison Redden and Kevin D. Moeller
Green Chem., 2011, Advance Article
DOI: 10.1039/C1GC15207F











![[4+2+1] domino cyclisation in water](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C1GC15183E)