© CNRS Photothèque / Cyril FRESILLON
Franck Dumeignil is a Professor in the Unit of Catalysis and Solid State Chemistry at the University of Lille, France. His research is focused on upgrading alcohols from biomass to obtain fuel, solvents and building blocks for the chemical industry, and enhancing bio-oil for energy needs. Franck took a few moments to talk to Green Chemistry…
Who or what initially inspired you to become a chemist?
I always liked science in general – mathematics, physics and chemistry. I must confess that I was a lazy student at that time, but I could obtain nice results in chemistry without working a lot, while for mathematics I had to work a lot harder to achieve good results! That was when I decided to select chemistry as a speciality. At University we had an impressive teacher, Prof. Ginette Leclercq, who was responsible for the catalysis lectures. Thanks to her I fell in love with catalysis and became a specialist in the subject! Ironically, after my PhD thesis in France and having spent almost 7 years in Japan, I came back to France, and obtained a full professor position due to an opening available because of the retirement of none other than Prof. Leclercq! I feel this is an amazing thing in my life – I could never have expected this when I was a student following the top-quality lectures of Prof. Leclercq, while being so impressed…
What has been the motivation behind your recent research?
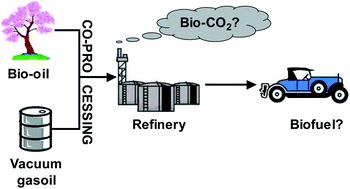
My recent research concerns catalysis for biorefineries. This is an exciting subject with so many perspectives, so many new things to discover and new processes to develop and implement!
What do you see as the main challenges facing research in this area?
There are multiple challenges. The molecules issued from biomass are more reactive than those issued from fossil resources. This can be seen as a decisive advantage, but when using heterogeneous catalysis, the catalysts then tend to coke much more rapidly. Furthermore, the feeds contain water, and the reactions generate water in most of the cases: what happens to the catalytic sites in these conditions? Another challenge is that the biomass-derived molecules usually contain a few different moieties, and selective attack is also an issue.
Where do you see the field of Green Chemistry being in 5 or 10 years time?
Green chemistry will develop and reach maturity as green metrics will be refined and become more and more reliable. What people usually need is ‘numbers’, which are much more concrete than concepts. Reliably and systematically quantifying green chemistry for any process/reaction will be a decisive advance in this field.
And finally…
If you could not be a scientist, but could be anything else, what would you be?
A pianist, or a composer (not too late!), or a F1 driver, or even a squash player! My hobbies are of course in line with this!
Take a look a couple of Franck’s recent articles in Green Chemistry – free to access until the 4th May 2012:
Selective catalytic oxidation of glycerol: perspectives for high value chemicals, Benjamin Katryniok, Hiroshi Kimura, Elżbieta Skrzyńska, Jean-Sébastien Girardon, Pascal Fongarland, Mickaël Capron, Rémy Ducoulombier, Naoki Mimura, Sébastien Paul and Franck Dumeignil, Green Chem., 2011, 13, 1960-1979
Glycerol dehydration to acrolein in the context of new uses of glycerol, Benjamin Katryniok, Sébastien Paul, Virginie Bellière-Baca, Patrick Rey and Franck Dumeignil, Green Chem., 2010, 12, 2079-2098
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.