Hydrogen peroxide (H2O2) could be synthesized directly from molecular H2 and O2 using a supported heteropolyacid catalyst and water as solvent at ambient temperature.
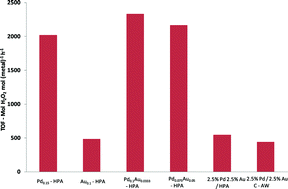
In this work, the Au-Pd exchanged supported Cs-heteropolyacid catalysts developed by Hutchings showed excellent H2O2 synthesis activity and were considerably more effective in achieving high yields than previously reported catalysts. By performing the reaction at ambient temperature in water in the absence of acid or halide additives, this method offers a potentially cleaner and more sustainable route to H2O2.
This article is free to access until 15th December 2011! Click the link below to find out more…
Direct synthesis of hydrogen peroxide using Au–Pd-exchanged and supported heteropolyacid catalysts at ambient temperature using water as solvent, Edwin N. Ntainjua, Marco Piccinini, Simon J. Freakley, James C. Pritchard, Jennifer K. Edwards, Albert F. Carley and Graham J. Hutchings, Green Chem., 2012, DOI: 10.1039/C1GC15863E










