A new recyclable copper catalyst has been developed which could be reused 20 times in the Diels-Alder reaction in ionic liquids without any obvious loss of activity or enantioselectivity.
Homogeneous metal-catalysed reactions are widely employed in organic synthesis. However, because most catalysts are expensive and because the metal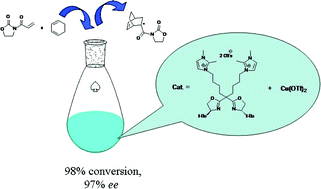
Zhi-Ming Zhou and colleagues from China report a series of imidazolium-tagged C2-symmetric bis(oxazoline) copper catalysts. These catalysts were applied to the Diels-Alder reaction and achieved high levels of activity and enantioselectivity. Due to the nature of the ligand, these catalysts were soluble in ionic liquids, including the hydrophobic [Bmim]NTf2, which enabled good recyclability. In fact, these catalysts could be recycled at least 20 times without showing an obvious loss in activity or enantioselectivity.
To read more, please click on the link below:
Recyclable copper catalysts based on imidazolium-tagged C2-symmetric bis(oxazoline) and their application in D–A reactions in ionic liquids, Zhi-Ming Zhou, Zhi-Huai Li, Xiao-Yan Hao, Xiao Dong, Xin Li, Li Dai, Ying-Qiang Liu, Jun Zhang, Hai-feng Huang, Xia Li and Jin-liang Wang, Green Chem., 2011, DOI: 10.1039/C1GC15788D
This article is free to access until the 14th October!










