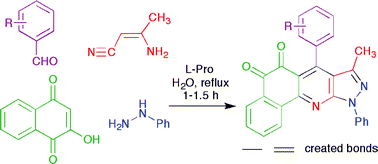
A four-component reaction catalysed by L-proline has been developed to efficiently synthesize a series of complex ortho-quinones.
Quinoline-detrived ortho-quinones are very important biological compounds and play crucial roles as cofactors, being involved in several biochemical reactions. Therefore there is interest in providing economical routes to these compounds. One strategy that could be employed are multi-comnponent reactions. However, little attention has been focused on the development of these reactions in aqueous conditions.
To read more, please click on the click below:
L-Proline-catalysed sequential four-component “on water” protocol for the synthesis of structurally complex heterocyclic ortho-quinones, Stephen Michael Rajesh, Balasubramanian Devi Bala, Subbu Perumal and J. Carlos Menéndez, Green Chem., 2011, DOI: 10.1039/C1GC15794A
This article is free to access until the 14th October 2011!










