Over the past 25 years, Green Chemistry has provided a unique forum for the publication of innovative research on the development of alternative sustainable technologies, efficient utilisation of resources and the concomitant minimisation of waste. We are delighted to bring together as very special issue containing articles by members of the green chemistry community as well as past and present Green Chemistry Board members, to mark and celebrate our first 25 years.
Among the contributions to this themed collection is a Paper on the application of a highly recyclable heterogeneous catalyst to the multi-decagram synthesis of enantiomerically enriched molecules in continuous-flow using a 2 mL reactor. The combination of this catalyst with a new reactor design increased the productivity from milligrams to up to 20 g scale in a few hours while reducing the overall environmental impact of the reaction (DOI: 10.1039/D4GC00019F)
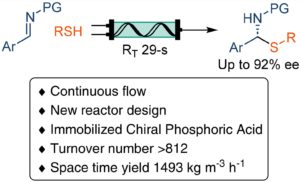 Organocatalysis has become one of the pillars of asymmetric catalysis along with metal and enzyme-catalyzed reactions. Its potential was recognized in 2019 by the IUPAC, as a part of the top 10 emerging technologies in chemistry, and by the Nobel Prize awarded to Benjamin List and David MacMillan in 2021.However, these reactions are often too inefficient due to the high catalyst cost and scalability issues, leading to limited applicability in the industry. In this context, developing heterogeneous catalysts can simplify the recycling process, making catalytic processes more efficient and contributing to minimizing the overall cost and environmental impact of the reaction.
Organocatalysis has become one of the pillars of asymmetric catalysis along with metal and enzyme-catalyzed reactions. Its potential was recognized in 2019 by the IUPAC, as a part of the top 10 emerging technologies in chemistry, and by the Nobel Prize awarded to Benjamin List and David MacMillan in 2021.However, these reactions are often too inefficient due to the high catalyst cost and scalability issues, leading to limited applicability in the industry. In this context, developing heterogeneous catalysts can simplify the recycling process, making catalytic processes more efficient and contributing to minimizing the overall cost and environmental impact of the reaction.
Read our interview with Aitor Maestro and C. Oliver Kappe
What is the motivation behind this work?
Although the use of heterogeneous catalysts and continuous flow technology in asymmetric catalysis is not new, the productivity of known processes is often rather limited. Developing new and reliable enantioselective processes for reproducing batch reactions on a large scale requires a combination of chemical and technical solutions. In this article, we wanted to illustrate the potential of combining these techniques to achieve higher productivity and set up a precedent for future developments in the field.
What aspects of this work are you most excited about at the moment and what do you find most challenging about it?
One of the most exciting results of this project was the high robustness of the heterogeneous catalyst. While similar homogeneous reactions often require 2-10 mol% of the catalyst, we managed to decrease it up to 0.1%. Moreover, the analysis of the catalyst after the reaction did not show any visible physical or chemical degradation, indicating it could be used for much longer.
One of the biggest challenges is the development of new heterogeneous catalysts that efficiently mimic the behaviour of their homogeneous counterparts. While solid supports facilitate the recycling process, they can also affect the reactivity.
What is the next step? What work is planned?
We are willing to further explore the potential of highly active heterogeneous chiral catalysts for other applications in the future. We want to apply them to the synthesis of key chiral building blocks and active pharmaceutical ingredients.
Please describe your journey to becoming part of the Green Chemistry community
Integrating technical solutions with intricate catalytic reactions helps reduce the overall waste, create more sustainable chemical processes, and enhance reaction efficiency. Therefore, focusing on the intersection of catalysis and technology aligns perfectly with the principles of green chemistry.
Why did you choose to publish in Green Chemistry?
This is one of the leading journals in the field of green chemistry research. We thought our work involving catalyst recyclability studies and waste reduction of the reported enantioselective reaction was a good fit for the scope of the journal.
What do you think the Green Chemistry journal has done well in the past 25 years, and what do you think are the main challenges our community will face in the next 25 years?
One of the biggest challenges for the journal is being focused on a constantly changing field. Many aspects of green chemistry are often directed by global trends or new legal regulations. Some current challenges that will probably get more attention in the coming years are obtaining raw chemicals from renewable sources such as CO2 or the development of economically viable catalytic processes.
Meet the corresponding authors
 |
Aitor Maestro obtained his PhD (2019) from the University of the Basque Country, working on asymmetric organocatalysis. After two postdoctoral stays (University of Groningen, and University of St. Andrews), in 2022, he obtained a Postdoctoral Fellowship from the Basque Government, allowing him to spend two years at the University of Graz working on asymmetric catalysis in continuous flow before moving back to the University of the Basque Country, where he is currently working on independent projects related to the development and applications of recyclable chiral catalysts. |
 |
Oliver Kappe received his diploma (1989) and his doctoral (1992) degrees in organic chemistry from the University of Graz and after two postdoctoral stays (University of Queensland and Emory University) returned to Graz in 1996 to start his indepenent acacdemic career and was appointed Full Professor in 2011. For the past decade the focus of his research has been directed towards flow chemistry/microreaction technology, encompassing a wide variety of synthetic transformations and experimental techniques. |











