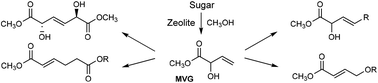
The metathesis and Claisen chemistry of methyl vinyl glycolate
Danish scientists have highlighted the diverse chemistry methyl vinyl glycolate (MVG) – a byproduct of the stannosilicate-catalysed formation of methyl lactate from monosaccharides and disaccharides – can offer as a powerful green platform molecule.
Robert Madsen and colleagues at the Technical University of Denmark used Grubbs-type catalysts to dimerise MVG by homo metathesis to yield (E)-2,5-dihydroxyhex-3-enedioate, a diastereomeric diester with potential for use as a monomer in the synthesis of functionalised polyesters. Cross metathesis of MVG with long chain terminal olefins gave unsaturated fatty acid methyl esters, which may be hydrogenated into customisable surfactants.
Performing Claisen-type rearrangements, the team also obtained industrially important unsaturated derivatives of adipic acid – a starting material for synthetic fibres. Palladium-catalysed transposition of MVG’s allylic alcohol derivative also resulted in a 1,4-dioxygenated motif which could be used as a precursor to 1,4-butanediol or γ-butyrolactone.
Want to know more? Read this article online, which is free to access until 30 September 2016:
“Methyl vinyl glycolate as a diverse platform molecule” by A. Sølvhøj et al., Green Chem., 2016, DOI: 10.1039/C6GC01556E










