Cross coupling reactions are immensely useful in the construction of organic molecules, a fact recognised with the award of the 2010 Nobel Prize in chemistry to Akira Suzuki, Richard Heck, and Ei-ichi Negishi. Now scientists in Beijing have discovered that adding common salts like sodium chloride, in a large excess, to Pd/C catalysed Suzuki-Miyaura reactions can more than double the achievable yield. This use of cheap and abundant materials would appear to be an excellent way to improve the productivity of the reaction without drastically increasing its burden on the environment.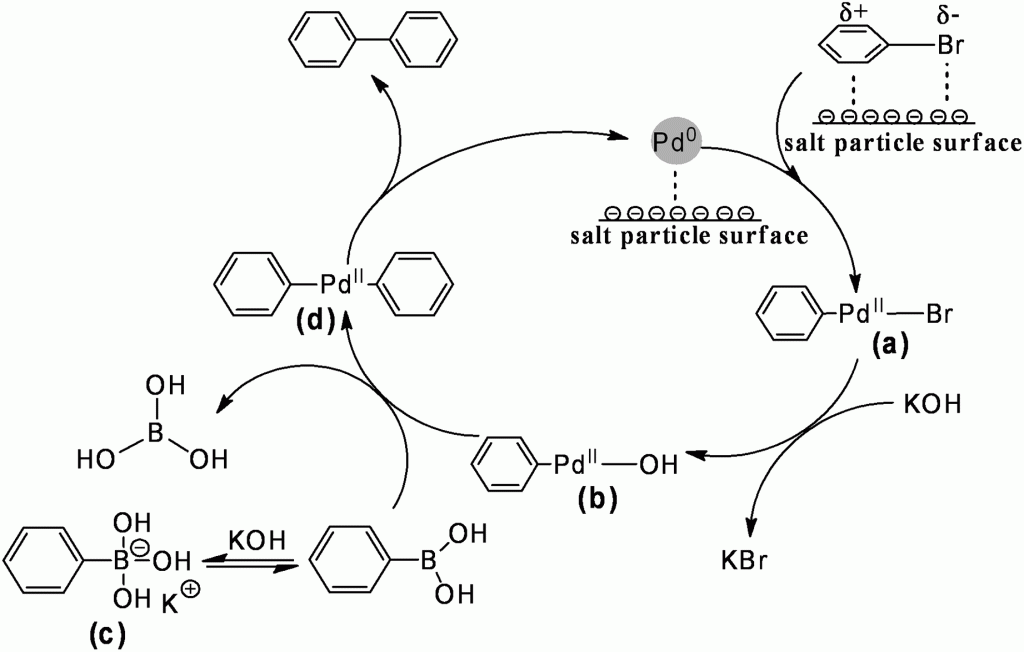
It is known that Suzuki-Miyaura reactions can be conducted without any catalyst specifically being added to the reaction. Instead, the reaction is catalysed by trace amounts of palladium in the inorganic base. The authors of this present work report that the inorganic salts are not catalytic themselves, but enhance the activity of the true catalyst. The nature of this synergetic effect was deduced from infra-red and X-ray photoelectron spectroscopy studies, showing evidence of the auxiliary salt interacting with both the bromoarene reactant and the palladium catalyst. The polarisation of the reactive C-Br bond, and the increased electron density placed on the metal catalyst, is said to enhance the rate determining oxidative addition step of the reaction.
By James Sherwood
Click below to read the full article, free until December 1st:
Acceleration of Suzuki coupling reaction by abundant and non-toxic salt particles, Binbin Zhang, Jinliang Song, Huizhen Liu, Jinghua Shi, Jun Ma, Honglei Fan, Weitao Wang, Peng Zhang and Buxing Han, Green Chem., 2013, DOI: 10.1039/C3GC42088D










