Albert Matlack, one of the founding educators of green chemistry and author of the seminal book ‘Introduction to Green Chemistry’, selects his favourite papers in Green Chemistry this month…
Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations, Shun Nishimura, Atsushi Takagaki and Kohki Ebitani, Green Chem., 2013, 15(8),2026–2042, DOI: 10.1039/C3GC40405F
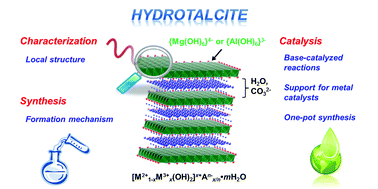 This is a review of hydrotalcites, which are layered magnesium aluminum hydroxy carbonates. They are the strongest bases known and are easy to recover for reuse by filtration. They are known for improved selectivity and yield when reactions are run in the constrained space between layers. Zeolites, metal organic frameworks and clays also offer such advantages. The review covers the synthesis, reactions alone and when used as a support for transition metals.
This is a review of hydrotalcites, which are layered magnesium aluminum hydroxy carbonates. They are the strongest bases known and are easy to recover for reuse by filtration. They are known for improved selectivity and yield when reactions are run in the constrained space between layers. Zeolites, metal organic frameworks and clays also offer such advantages. The review covers the synthesis, reactions alone and when used as a support for transition metals.
*Open Access* A prototype device for evaporation in batch and flow chemical processes, Benjamin J. Deadman, Claudio Battilocchio, Eric Sliwinskia and Steven V. Ley, Green Chem., 2013, 15(8), 2050–2055, DOI: 10.1039/C3GC40967H
 In this work, Ley et. al. have developed a device for evaporating, concentrating and switching solvents in continuous flow so that all solvents can be recovered for reuse. Twenty one solvents ranging from dichloromethane to dimethylformamide were recovered satisfactorily. This enlarges the possibilities for the use of microchanneled reactors in process intensification. They offer higher selectivities and yields, safer reactions, easy heating and cooling, no need to worry about explosive limits, no need for a pilot plant with smaller cheaper chemical plants. Other methods include spinning disc and tube in tube reactors. With the latter, up to 15 tons per hour can be processed. Oxford Catalysts has developed such systems for steaming reforming plus Fischer-Tropsch conversion of stranded natural gas to liquids. Two research groups have developed microchanneled reactors with inline analysis for five variables that are self-optimizing over a period of two to three. The group of Buchwald did it for a Heck Reaction and Poliakoff‘s group did for the reaction of 1-pentanol with dimethyl carbonate.
In this work, Ley et. al. have developed a device for evaporating, concentrating and switching solvents in continuous flow so that all solvents can be recovered for reuse. Twenty one solvents ranging from dichloromethane to dimethylformamide were recovered satisfactorily. This enlarges the possibilities for the use of microchanneled reactors in process intensification. They offer higher selectivities and yields, safer reactions, easy heating and cooling, no need to worry about explosive limits, no need for a pilot plant with smaller cheaper chemical plants. Other methods include spinning disc and tube in tube reactors. With the latter, up to 15 tons per hour can be processed. Oxford Catalysts has developed such systems for steaming reforming plus Fischer-Tropsch conversion of stranded natural gas to liquids. Two research groups have developed microchanneled reactors with inline analysis for five variables that are self-optimizing over a period of two to three. The group of Buchwald did it for a Heck Reaction and Poliakoff‘s group did for the reaction of 1-pentanol with dimethyl carbonate.
Highly efficient production of lactic acid from cellulose using lanthanide triflate catalysts, Fen-Fen Wang, Chun-Ling Liu and Wen-Sheng Dong, Green Chem., 2013, 15(8), 2091–2095, DOI: 10.1039/C3GC40836A
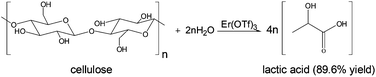 In this work, cellulose was converted to lactic acid with an erbium triflate catalyst in water at 240 degrees C, in 30 minutes in 89.6% yield. The catalyst showed no loss of activity after five runs. This avoids the need to hydrolyze cellulose to sugars as a separate step. Lactic acid is the monomer for the commercial poly(lactic acid) which is biodegradable. The conversion of lactic acid to acrylic acid is being commercialized.
In this work, cellulose was converted to lactic acid with an erbium triflate catalyst in water at 240 degrees C, in 30 minutes in 89.6% yield. The catalyst showed no loss of activity after five runs. This avoids the need to hydrolyze cellulose to sugars as a separate step. Lactic acid is the monomer for the commercial poly(lactic acid) which is biodegradable. The conversion of lactic acid to acrylic acid is being commercialized.










