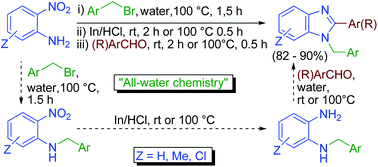
Scientists from India have developed a tandem N-alkylation-reduction-condensation route to synthesize N-arylmethyl-2-substituted benzimidazoles.
1,2-Disubstituted benzimidazoles exhibit a broad range of biological activities, which make them a very popular synthetic target. The regio-specific synthesis of these compounds is the main synthetic challenge, with the desired regioselectivity of the final compounds determined at the aryl-amination stage. However, this traditionally requires a transition metal catalyst, expensive ligands and an appropriate base.
Read the full article for free until the 4th December 2012!
“All-water” chemistry of tandem N-alkylation–reduction–condensation for synthesis of N-arylmethyl-2-substituted benzimidazoles, Damodara N. Kommi, Dinesh Kumar, Rohit Bansal, Rajesh Chebolu and Asit K. Chakraborti, Green Chem., 2012, DOI: 10.1039/C2GC36377A
You may also be interested in this article as well – free to access for two weeks:
Catalytic procedures for multicomponent synthesis of imidazoles: selectivity control during the competitive formation of tri- and tetrasubstituted imidazoles, Dinesh Kumar, Damodara N. Kommi, Narendra Bollineni, Alpesh R. Patel and Asit K. Chakraborti, Green Chem., 2012, 14, 2038-2049
Stay up-to-date with the latest news and content in Green Chemistry by registering for our free table of contents alerts.










