Oxidation represents one of the most important reactions in organic synthesis and looks to have a significant role on the development and synthesis of value-added chemicals from biomass. Efforts to make oxidation reactions more sustainable have led to the development of heterogeneous catalysts and the use of molecular oxygen an alternative to traditional, toxic chemical oxidants.
Read this article for free until the 29th November 2012!
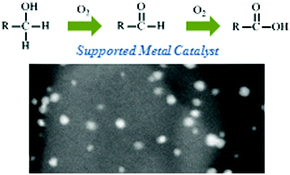
Selective oxidation of alcohols and aldehydes over supported metal nanoparticles, Sara E. Davis, Matthew S. Ide and Robert J. Davis, Green Chem., 2012, DOI: 10.1039/C2GC36441G
You may also be interested in these related articles – free to access until the 15th November 2012:
On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts, Sara E. Davis, Bhushan N. Zope and Robert J. Davis, Green Chem., 2012, 14, 143-147
Inhibition of gold and platinum catalysts by reactive intermediates produced in the selective oxidation of alcohols in liquid water, Bhushan N. Zope and Robert J. Davis, Green Chem., 2011, 13, 3484-3491
Stay up-to-date with the latest news and content in Green Chemistry by registering for our free table of contents alerts.










