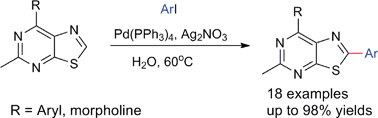
Chinese scientists have developed a new method for synthesising 2-arylsubstituted thiazolo[5,4-d]pyrimidine derivatives under mild conditions.
This article is free to access until the 6th July 2012! Click on the link below to find out more…
“On water” direct Pd-catalysed C–H arylation of thiazolo[5,4-d]pyrimidine derivatives, Ye-Xiang Su, Ya-Hui Deng, Ting-Ting Ma, Yu-Yan Li and Li-Ping Sun, Green Chem., 2012, DOI: 10.1039/C2GC35399G
You may also be interested in these articles – free to access for 2 weeks:
Efficient and convenient C-3 functionalization of indoles through Ce(OAc)3/TBHP-mediated oxidative C–H bond activation in the presence of β-cyclodextrin, Yu Lin Hu, Hui Jiang and Ming Lu, Green Chem., 2011, 13, 3079-3087
Greener solvents for ruthenium and palladium-catalysed aromatic C–H bond functionalisation, Cedric Fischmeister and Henri Doucet, Green Chem., 2011, 13, 741-753
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.










