A supported organaocatalyst has been developed for direct asymmetric Aldol reaction in water with higher enantioselectivity than the original homogeneous catalyst.
Asymmetric organocatalyst is considered to be a very powerful tool for stereoselective synthesis on enantiomerically enriched compounds. However, organocatalytic reactions, whilst avoiding many of the problems associated with metal catalysts, often require high catalyst loadings and tedious purification of the products.
This article is free to access until the 24th May 2012! Click on the link below to find out more…
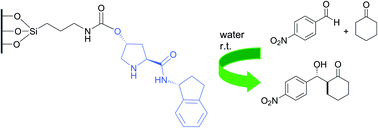
Recyclable silica-supported prolinamide organocatalysts for direct asymmetric Aldol reaction in water, Amàlia Monge-Marcet, Xavier Cattoën, Diego A. Alonso, Carmen Nájera, Michel Wong Chi Man and Roser Pleixats, Green Chem., 2012, DOI: 10.1039/C2GC35227C
You may also be interested in the following article as well – free to access for 2 weeks!
Advances in catalytic metal-free reductions: from bio-inspired concepts to applications in the organocatalytic synthesis of pharmaceuticals and natural products, Magnus Rueping, Jeremy Dufour and Fenja R. Schoepke, Green Chem., 2011, 13, 1084-1105
Stay up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.










