In this critical review, Karen Connelly and Hicham Idriss from the University of Aberdeen, UK and SABIC T&I, Saudi Aradia, review the fundamental aspects behind the reactivity of TiO2 surfaces.
This article is free to access until the 11th January 2012! Click the link below to read more…
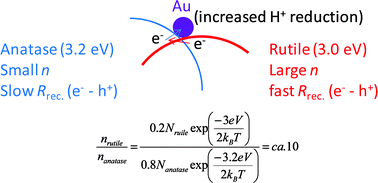
The photoreaction of TiO2and Au/TiO2single crystal and powder surfaces with organic adsorbates. Emphasis on hydrogen production from renewables, Karen A. Connelly and Hicham Idriss, Green Chem., 2012, DOI: 10.1039/C1GC15992E
You may also be interested in this article, also free until the 11th January 2012:
Sustainable hydrogen production by the application of ambient temperature photocatalysis, Michael Bowker, Green Chem., 2011, 13, 2235-2246










