Scientists from Germany have developed an efficient diastereoselective synthesis of 2,5-disubstituted tetrahydrofurans in three steps.
Magnus Rueping and Vilas Phapale used [IrCl(cod)]2 to catalyze the α-alkylation of substituted acetophenones with 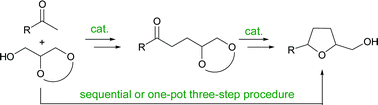
Furthermore, a one-pot, three-step version of this procedure could be performed which does not require chomatographic purification of any of the intermediates.
This article is free to access until the 22nd November 2011! To read more, please click the link below…
Effective synthesis of 2,5-disubstituted tetrahydrofurans from glycerol by catalytic alkylation of ketones, Magnus Rueping and Vilas B. Phapale, Green Chem., 2011, DOI: 10.1039/C1GC15764G










