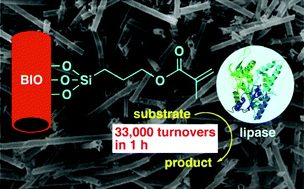
Lipases immobilized onto biogenous iron oxide via an organic bridging group were applied to the kinetic resolution of secondary alcohols and could be used up to 5 times.
Immobilization of enzymes helps to achieve cost-effective, clean biocatalysis. However, the method of immobilization employed can have a big impact on the catalytic activity, selectivity, thermostability and recyclability of enzymes.
Scientists from Japan have used biogenous iron oxide (BIO) from iron-oxidising bacteria as a support for enzymes. BIO was chemically modified with silane coupling agents to allow immobilization of the enzyme. The supported catalyst was then applied to the resolution of secondary alcohols and could achieve a turnover frequency of 33 000 h-1. Heat treatment of BIO before chemical modification generated magnetic-BIO which was also employed as a support, and the immobilized enzymes could be recovered easily by using a magnet.
This article is free to access until 22nd November! Click the link below to read more…
Highly active lipase immobilized on biogenous iron oxide via an organic bridging group: the dramatic effect of the immobilization support on enzymatic function, Tadashi Ema, Yuki Miyazaki, Izumi Kozuki, Takashi Sakai, Hideki Hashimoto and Jun Takada, Green Chem., 2011, DOI: 10.1039/C1GC15877E