 A recent Green Chemistry article from Julie Zimmerman, Paul Anastas and co-workers from Yale University and Baylor University describes guidelines which should be followed in order to design chemicals with reduced aquatic toxicity. Their article has been highlighted in Nature News.
A recent Green Chemistry article from Julie Zimmerman, Paul Anastas and co-workers from Yale University and Baylor University describes guidelines which should be followed in order to design chemicals with reduced aquatic toxicity. Their article has been highlighted in Nature News.
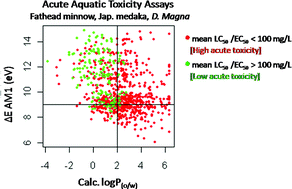
The team highlight that there is a need for synthetic chemists to focus on design of safer chemicals rather than testing for toxicity after production. The team explored mechanistically-driven qualitative and quantitative analyses between the in-silico predicted molecular properties and in vivo toxicity data to propose property limits associated with higher probabilities of safe chemicals. They propose design guidelines that can be used to significantly increase the probability that a chemical will have low toxicity to the aquatic species studied.
Interested in knowing more? Read the full article for free until September 1st!
Towards rational molecular design: derivation of property guidelines for reduced acute aquatic toxicity
Adelina M. Voutchkova, Jakub Kostal, Justin B. Steinfeld, John W. Emerson, Bryan W. Brooks, Paul Anastas and Julie B. Zimmerman
Green Chem., 2011, DOI: 10.1039/C1GC15651A