The photocatalyst ZnIn4S4was synthesized hydrothermally and applied to the conversion of hydrogen sulfide to hydrogen under solar light.
Scientists from India have demonstrated that a zinc nanostructured photocatalyst could be used to decompose 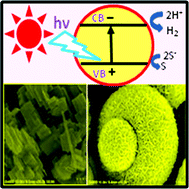
Kale and colleagues report here the controlled synthesis of ZnIn4S4by a hydrothermal method and its application to H2S conversion to H2 using solar energy. The H2 evolution rate obtained is much higher compared to earlier reported photocatalysts.
To read more, just click on the link below. This article is free to access until the 21st September 2011!
Ecofriendly hydrogen production from abundant hydrogen sulfide using solar light-driven hierarchical nanostructured ZnIn2S4photocatalyst, Nilima S. Chaudhari, Ashwini P. Bhirud, Ravindra S. Sonawane, Latesh K. Nikam, Sambhaji S. Warule, Vilas H. Rane and Bharat B. Kale, Green Chem., 2011, DOI: 10.1039/C1GC15515F










