Hydrolytic hydrogenation of cellulose with hydrotreated caesium salts of heteropoly acids and Ru/C. 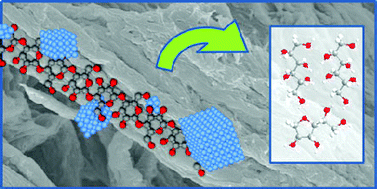
However, in this work Sels and co-workers have found that the caesium salts of HPAs are not only highly selective (giving up to 90% yields of hexitols) and can be performed under mild reaction conditions, the Cs HPA salts could be recovered by simple recrystallisation at room temperature without using organic solvents. (Green Chem., 2011, DOI: 10.1039/c1gc15350a)
Selective oxidation of 5-hydroxymethyl-2-furfural using supported gold-copper nanoparticles. 5-Hydroxymethyl-2-furfural (HMF), formed from the dehydration of sugars, can be oxidised to 2,5-furandicarboxylic acid (FDCA), which recently has been suggested as a substitute for terephthalate acid – the monomer for the 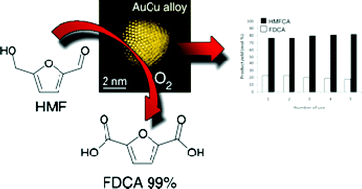
In this work Hutchings and colleagues report the use of gold-copper supported nanoparticles as an effective catalyst for the oxidation of HMF to FDCA. Although supported gold nanoparticles have been applied to this reaction previously, catalyst stability has remained very low. However, the bimetallic nanoparticles reported here, supported on titania, exhibit a remarkable degree of stability, even in the presence of base. The catalyst could be recovered by filtration and reused several times without significant loss of activity. (Green Chem., 2011, DOI: c1gc15355b)










