Production of liquid hydrocarbon fuels by catalytic conversion of biomass-derived levulinic acid. Dumesic and co-workers report the use of a highly active bimetallic catalyst for the conversion of levuilinic acid to γ-valerolactone (GVL). The RuRe/C catalyst used in this work is significantly more active that the traditional Ru/C, and shows stable activity and stereoselectivity in the presence of sulfuric acid. A model techno-economical analysis has shown that this new calaytic process can be integrated with the catalyic conversion of GVL to butene, followed by alkene oligomerization, and could provide a cost effective route for converting biomass into liquid hydrocarbon fuels. (Green Chem., 2011, DOI:10.1039/c1gc15047b)
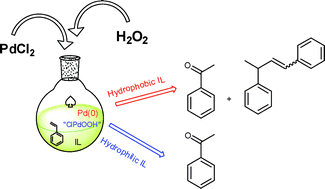
Styrene oxidation by hydrogen peroxide in ionic liquids: the role of the solvent on the competition between two Pd-catalyzed processes, oxidation and dimerization. In this work, Chiappe and her team investigated the role of solvent in the Wacker oxidation of styrene, in ionic liquids (ILs). They found that the nature of the IL has a strong influence on the outcome of the reaction. In the hydrophillic ILs, a homogeneous solution is formed and gives the expected product with high selectivity, and only a small amount of by-products. However, in hydrophobic ILs, the aqueous solution of hydrogen peroxide is immiscible and so forms a biphasic system. In this case side reactions occur, and the palladium catalyst is reduced from Pd(II) to Pd(0) and reoxidation back to Pd(II) is disfavoured. (Green Chem., 2011, DOI:10.1039/c0gc00945h)
Read these articles for free until 24th June