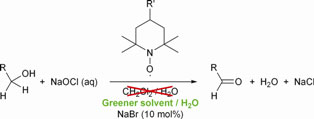
Scientists from the Netherlands and the UK have conducted a solvent screening study aimed at identifying greener alternatives for the commonly used solvent dichloromethane in N-oxy catalysed bleach oxidations of various alcohol substrates.
The team found that environmentally acceptable ester solvents, notably isopropyl acetate and methyl acetate, gave results comparable to or better than dichloromethane.
However, there was no apparent correlation between common solvent properties and performance.
A comparison of two co-catalysts, NaBr and borax, revealed that borax gave better results with cinnamyl alcohols whereas NaBr was generally better with the other alcohols.
The team also studied the effect of catalyst loading. In the oxidation of 3-phenyl-1-propanol the amount of N-oxy catalyst could be effectively reduced to a mere 0.1 mol%.
They concluded that due to the complex nature of these systems, there is not a single set of conditions that gives good results for all alcohols. However, by employing a simple screening approach to assess solvent, catalyst and co-catalyst combinations, similar or even better results can often be achieved in solvents other than dichloromethane.
Read more about this article:
Towards greener solvents for the bleach oxidation of alcohols catalysed by stable N-oxy radicals
M H A Janssen, J F Chesa Castellana, H Jackman, P J Dunn and R A Sheldon, Green Chem., 2011, DOI:10.1039/c0gc00684j