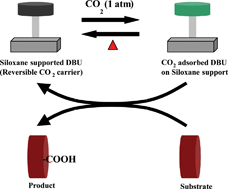
Beckman and Munshi reports a reversible CO2 carrier (RCC) for the carboxylation of ketone to β-ketoester under ambient CO2 pressure and temperature.
RCC has been synthesized by immobilizing 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) on methylhydrosiloxane support and reacting with CO2 with 100% degree of functionalisation. RCC is found to be recyclable and shows retention of activity in 5 recycles. CO2 absorption under ambient temperature and desorption at 120 °C renders the material suitable for carrying out carboxylation reactions at 25 °C with excellent yields. The yield of the reaction can reach up to 100% with TON 200 in 4 h. The extent of the reaction primarily depends upon enol content of the substrate. β-Ketoacid produced during the reaction can be isolated and converted to its corresponding methyl ester derivative by reacting with methyl iodide.
Access this article for free by clicking on the link below:
Ambient carboxylation on a supported reversible CO2 carrier: ketone to β-keto ester, Eric J. Beckman and Pradip Munshi, Green Chem., 2011, DOI: 10.1039/C0GC00704H, Advanced Article, Paper