Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to <it>Daphnia magna</it>
Jared S. Bozich, Samuel E. Lohse, Marco D. Torelli, Catherine J. Murphy, Robert J. Hamers and Rebecca D. Klaper
Environ. Sci.: Nano, 2014,1, 260-270
DOI: 10.1039/C4EN00006D
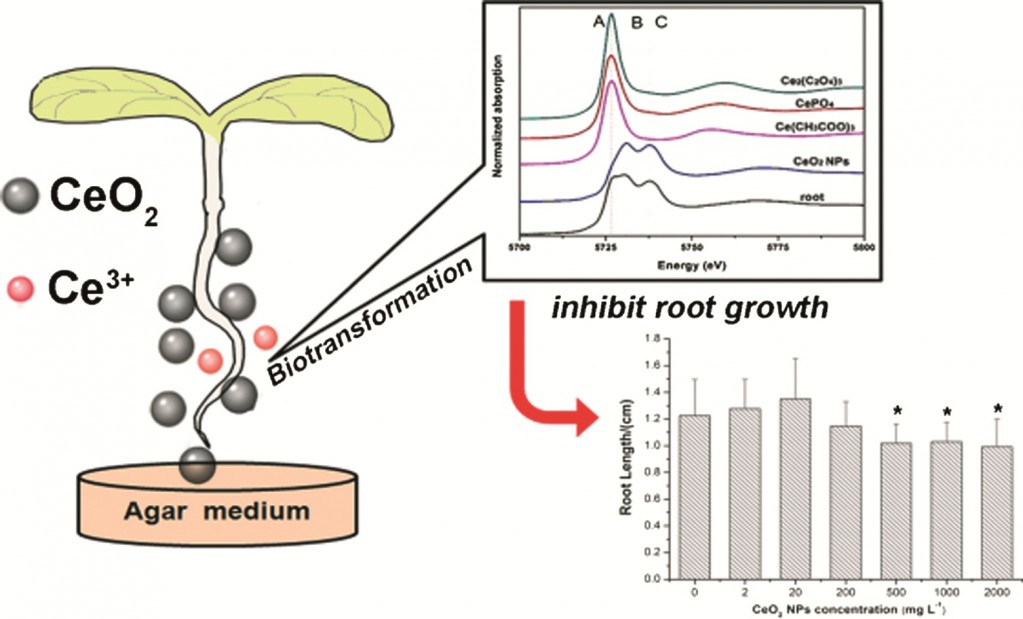
Recent advances in BiOX (X = Cl, Br and I) photocatalysts: synthesis, modification, facet effects and mechanisms
Liqun Ye, Yurong Su, Xiaoli Jin, Haiquan Xie and Can Zhang
Environ. Sci.: Nano, 2014,1, 90-112
DOI: 10.1039/C3EN00098B
Zeolite and mesoporous silica nanomaterials: greener syntheses, environmental applications and biological toxicity
Sean E. Lehman and Sarah C. Larsen
Environ. Sci.: Nano, 2014,1, 200-213
DOI: 10.1039/C4EN00031E
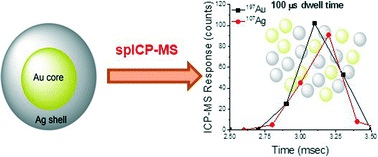
Tracking dissolution of silver nanoparticles at environmentally relevant concentrations in laboratory, natural, and processed waters using single particle ICP-MS (spICP-MS)
D. M. Mitrano, J. F. Ranville, A. Bednar, K. Kazor, A. S. Hering and C. P. Higgins
Environ. Sci.: Nano, 2014,1, 248-259
DOI: 10.1039/C3EN00108C
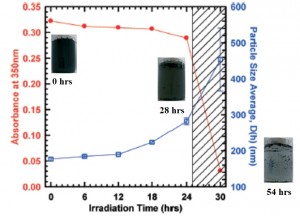
Synthesis and characterization of isotopically labeled silver nanoparticles for tracing studies
Adam Laycock, Björn Stolpe, Isabella Römer, Agnieszka Dybowska, Eugenia Valsami-Jones, Jamie R. Lead and Mark Rehkämper
Environ. Sci.: Nano, 2014,1, 271-283
DOI: 10.1039/C3EN00100H
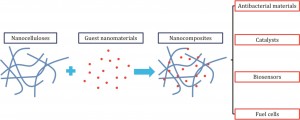
Green synthesis and formation mechanism of cellulose nanocrystal-supported gold nanoparticles with enhanced catalytic performance
Xiaodong Wu, Canhui Lu, Zehang Zhou, Guiping Yuan, Rui Xiong and Xinxing Zhang
Environ. Sci.: Nano, 2014,1, 71-79
DOI: 10.1039/C3EN00066D
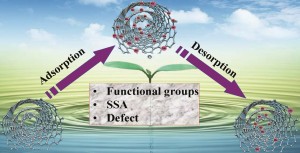
Localized fluorescent complexation enables rapid monitoring of airborne nanoparticles
Fanxu Meng, Maria D. King, Yassin A. Hassan and Victor M. Ugaz
Environ. Sci.: Nano, 2014,1, 358-366
DOI: 10.1039/C4EN00017J
Deposition of nanoparticles onto polysaccharide-coated surfaces: implications for nanoparticle–biofilm interactions
Kaoru Ikuma, Andrew S. Madden, Alan W. Decho and Boris L. T. Lau
Environ. Sci.: Nano, 2014,1, 117-122
DOI: 10.1039/C3EN00075C
Silver nanoparticle protein corona composition compared across engineered particle properties and environmentally relevant reaction conditions
Richard Eigenheer, Erick R. Castellanos, Meagan Y. Nakamoto, Kyle T. Gerner, Alyssa M. Lampe and Korin E. Wheeler
Environ. Sci.: Nano, 2014,1, 238-247
DOI: 10.1039/C4EN00002A
Bioavailability of inorganic nanoparticles to planktonic bacteria and aquatic microalgae in freshwater
Nadia von Moos, Paul Bowen and Vera I. Slaveykova
Environ. Sci.: Nano, 2014,1, 214-232
DOI: 10.1039/C3EN00054K
Take a look at the articles today and blog your thoughts and comments below.
Fancy submitting an article to Environmental Science: Nano? Then why not submit to us today or alternatively email us your suggestions.