The answer is nanomaterials. Particularly Carbon Black (CB) and silica nanomaterials which reinforce rubber increasing its durability, road grip and mileage. Other materials, such as carbon nanotubes (CNTs), offer better performance and ecological benefits via fuel savings. However, these are more expensive therefore their use is still very limited.
Nanomaterial fragments are generally released into the environment, constituting a rather uncontrollable source of emission of nanocomposites. In some countries where release quantification is already required, these emissions have been estimated between 4,000 and 7,000 tons of microplastic fragments. In spite of this immense environmental impact, little is known regarding the release from nanocomposites under mechanical and chemical stresses combined. Challenging the hypothesis of release being induced by a synergy of stresses, Wohlleben and co-workers bring a new sequence to test degradation pathways (Figure 1) as well as a fresh look at appropriate analytical techniques.
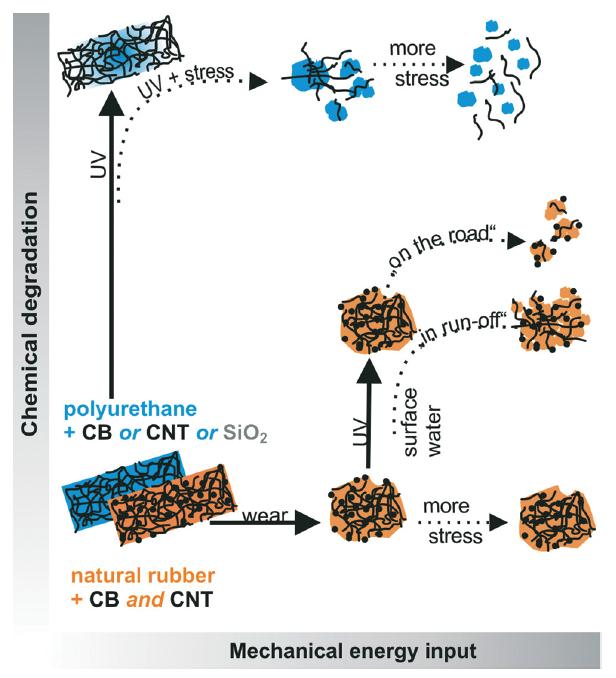
Figure 1. Synergetic degradation pathways by combined mechanical and chemical stresses.
In both cases shown in Figure 1, only chemical degradation or mechanical shear does not induce appreciable release of fragments. This happens only when the second stress is introduced, showing that synergetic degradation occurs on the diagonal of the scheme shown above.
In the first case, polyurethane (PU) with different single fillers (blue pathway in Figure 1) was first aged under standard conditions then put under mechanical stress simulating rain conditions (immersion, shaking or sonicating). Ultraviolet spectroscopy (UV-Vis), transmission electron microscopy (TEM) and analytical ultracentrifugation (AUC) or field flow fractionation were used to analyse the results. Images from X-ray photoelectron spectroscopy (XPS) showed that nanofillers remain on the surface after UV and rain weathering, accumulated into dense agglomerates as the polymer matrix was removed by the combined photolysis and hydrolysis.
Moreover, by creating an extended, highly reproducible, very low scatter semi-quantitative method to analyse turbidity of the released fragments, Wohlleben and co-workers were able to affirm that the release was reduced when CNTs were used (Figure 2a). Considering fragments below 150 nm diameter, PU filled with CNTs also showed reduced release (Figure 2b). More importantly, fragments coming from PU with CNTs were mostly organic, showing that the release of nanofiller fragments was suppressed.
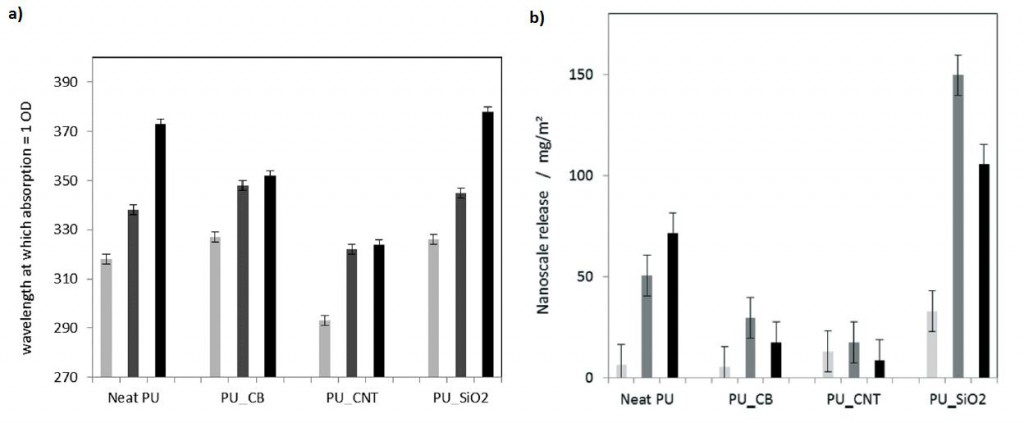
Figure 2. a) Turbidity assessment of the released fragments from aged PU surfaces after UV and rain, with increasing mechanical shear: 24 h immersion (light grey), 24 h shaking (dark grey) and 1 h sonicating (black). b) Size-selective analysis (AUC) of fragments in the size range of 5 nm to 150 nm released from aged PU surfaces after UV and rain, with increasing mechanical shear: 24 h immersion (light grey), 24 h shaking (dark grey) and 1 h sonicating (black).
In the second case in Figure 1, natural rubber (NR) nanocomposite were filled with 40% CB and 4% CNT representing an innovative reinforced tire tread and it was compared to NR with 40% CB representing a conventionally reinforced tread and also to neat NB. The test focused on a sequence of mechanical-chemical-mechanical stresses, enabling the simulation of dust aging on dry roads and also the direct run-off into surface waters of the secondary fragments using UV irradiation.
During sanding, there was no noticeable difference between the particle concentration of the three rubber specimen. After aging, the structural differences of the fragments were minimal between wet and dry aging. Moreover, being CB and CNT both relatively more inert to UV degradation, they seem to have accumulated on the surface (less oxidised organic structures were quantified).
Fragments could potentially release smaller fragments and even free nanomaterial. Hence, Wohlleben and colleagues also analysed this scenario and indeed, smaller fragments were formed when a second sanding process was introduced, with no significant differences between NB with both fillers and only CB. However, it clearly showed that dry aging induces stronger secondary fragmentation than submersed aging, these results being in contradiction with the expected combined effect of hydrolysis and photolysis being more aggressive than photolysis only.
In summary, regarding analytical techniques, simple UV-Vis was shown to be the most sensitive technique. Qualitative identification by TEM is essential and analysis of XPS images was also important for a plausibility check.
This study is the first to analyse the combined forces of mechanical fragmentation, environmental aging and again mechanical stresses, showing a stepwise sequence that could continue ad infinitum and be tailored to simulate specific scenarios and provide useful estimates of release rates, enabling more reliable modelling and risk assessments.
To read the full article for free* click the link below:
Release from nanomaterials during their use phase: combined mechanical and chemical stresses applied to simple and multi-filler nanocomposites mimicking wear of nano-reinforced tires
Wendel Wohlleben, Jessica Meyer, Julie Muller, Philipp Müller, Klaus Vilsmeier, Burkard Stahlmecke and Thomas A. J. Kuhlbusch
Environ. Sci.: Nano, 2016,3, 1036-1051
DOI: 10.1039/C6EN00094K, Paper

—————-
About the webwriter
Luiza Cruz is a PhD student in the Barrett Group at Imperial College London. Her work is towards the development of new medicines, using medicinal and
natural products chemistry.
—————-
*Access is free until 07/11/2016 through a registered publishing personal account.