Graphene oxide (GO) is a precursor material in the preparation of graphene. Despite its name, on the surface of this material there are different types of functional groups including epoxy, hydroxyl and carbonyl groups. As a result, GO is hydrophilic and easily dispersed in water. This has led to a variety of investigations relating to GO as a potential pollutant, as well as a possible treatment to cancer.
The disrupted π-bond structure in GO enables the absorption of significant amounts of light from solar radiation. Therefore, environmental processing of GO can be expected to include photochemical processes. One important outcome of such processing is the generation of reactive oxygen species (ROS). These ROS can include singlet oxygen (1O2), superoxide anions (O2.–) and hydroxyl radicals (.OH). Generation of ROS has a significant impact on ecological risks associated with GO and is critical in understanding the transformation pathways of carbon in the GO structure.
Therefore, Yingcan Zhao and Chad T. Jafvert at Purdue University (West Lafayette, USA) has investigated the ability of aqueous dispersions of single layered GO to generate ROS upon exposure to light within the solar spectrum (λ=300-410 nm). The generated ROS was detected using specific chemical probes, UV-vis spectroscopy and Raman spectroscopy.
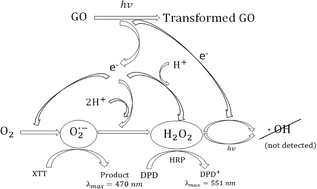
The findings of this research highlighted that upon exposure to solar radiation there is electron transfer reactions occurring from GO to dissolved O2, forming O2.– and significant quantities of H2O2.
Given the fact that these are reduction reactions, this resulted in an overall oxidation of GO. Some of the generated ROS reacted directly with the GO surface and therefore, the oxidation of GO was found to be non-stoichiometric.
The exposure to light also increased the chromophores content or the absorptivity of existing chromophores, as suggested by the increased darker colour of the GO suspensions. However, Raman spectroscopic analysis also indicated an increase in non-aromatic defects.
To access the full article, download a copy for free* by clicking the link below.
Environmental photochemistry of single layered graphene oxide in water
Yingcan Zhao and Chad T. Jafvert
Environ. Sci.: Nano, 2015, 2, 136-142
DOI: 10.1039/C4EN00209a
—————-
About the webwriter
Imali Mudunkotuwa is a Postdoctoral Scholar and Research Assistant at The University of Iowa. She is interested in nanoscience, physical and surface chemistry. You can find more articles by Imali in her author archive .
—————-
* Access is free until the 21/06/2015 through a registered RSC account