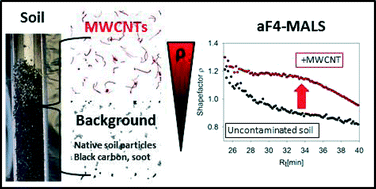
Multi-walled carbon nanotubes (MWCNT) are the preferred choice of nanotubes for many applications as they has a lower cost than the single walled carbon nanotubes (SWCNT). Therefore the chances of them getting into air, natural water systems and soil is extremely probable. Although many beneficial effects are postulated for MWCNTs direct applications such as incorporation into fertilizer to enhance water uptake, seed germination and cell growth can increase their levels in the environment, especially in soil. Since there is evidence of some negative affects on soil microbial communities as well as plants it is always better to have means of monitoring and controlling their levels in the environment. However, methods to detect and quantify MWCNTs in soil and sediments are still not well established. Therefore, Alexander Gogos and co-workers from the Agroscope, Institute for Sustainability Sciences in Switzerland have developed and evaluated a novel approach using asymmetric field flow fractionation (A4F) coupled with multi-angle light scattering (MALS) to differentiate MWCNTs in soil.
Here the high aspect ratios of MWCNT’s have been exploited to differentiate between MWCNTs, soot and native soil particles. The shape factors (ρ) for these materials were calculated by taking the ratio between the radius of gyration (rg) and the hydrodynamic radius (rh). Simply, the rg corresponds to the weighted average of all possible radii of a particle from its center of mass and rh is approximated for non-spherical particles as the radius of a sphere with same diffusion behavior. Elaborately, presence of MWCNTs in the mixtures resulted in increased ρ-values. The fractions of MWCNTs in the mixtures were calculated using the ρ-values obtained from A4F-MALS. They were cross-validated by comparing with the results obtained from automated electron microscopy analysis and were found to be in reasonable agreement. Since natural soils exhibited lower ρ-values consistently this method can be used in specific identification of MWCNTs as well as other high aspect ratio nanomaterials in soil.
To access the full article, download a copy for free* by clicking the link below.
Capabilities of asymmetric flow field-flow fractionation coupled to multi-angle light scattering to detect carbon nanotubes in soot and soil.
Alexander Gogos, Ralf Kaegi, Renato Zenobi, Thomas D. Bucheli
DOI: 10.1039/C4EN00070F
*Access is free through a registered RSC account – click here to register