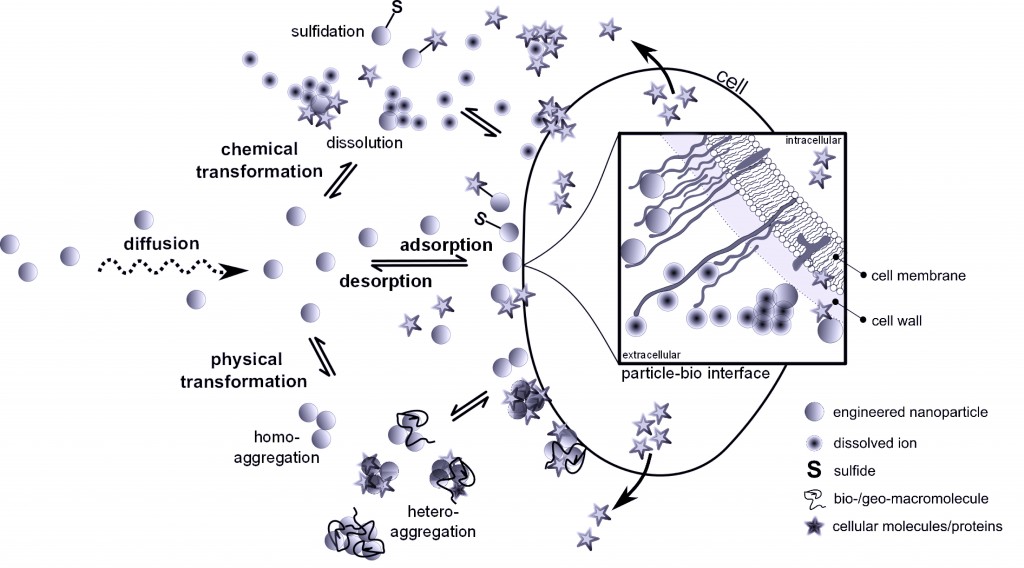
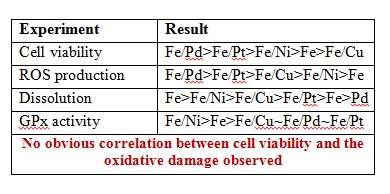
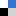Researchers are continuously on the lookout for materials which are more environmentally friendly and sustainable in order to improve emerging technologies. Nanoparticles (NPs) are the basis for a variety of emerging technologies used for industrial, biomedical and environmental applications, but their release into the environment is still a cause for concern. What if a nanomaterial, that has minimal negative environmental impact, was available? Jared Bozich from the University of Wisconsin and colleagues have published a research paper demonstrating that surface chemistry has the potential to increase or decrease negative biological impacts of NPs.
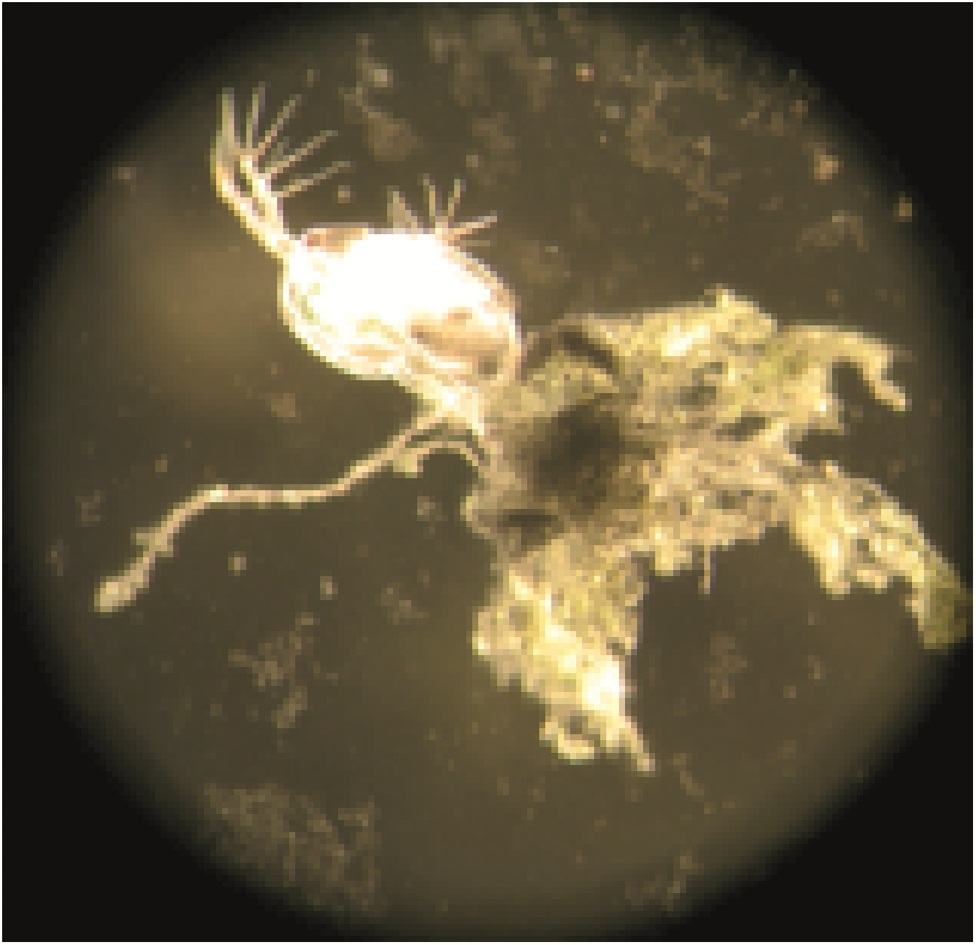 The surfaces of NPs are typically modified with surface functional groups that control properties such as stability. In this research, the acute and chronic toxicity of well characterized gold nanoparticles (AuNPs), functionalized with ligands of differing charges were investigated in Daphnia magna. D. magna (more commonly called water fleas) are widely accepted as a model organism for assessing the toxicity of environmental contaminates and experience reduced reproduction, growth and increased mortality with exposure to toxic substances. D.magna were exposed to concentrations of four types of functionalized AuNps.
The surfaces of NPs are typically modified with surface functional groups that control properties such as stability. In this research, the acute and chronic toxicity of well characterized gold nanoparticles (AuNPs), functionalized with ligands of differing charges were investigated in Daphnia magna. D. magna (more commonly called water fleas) are widely accepted as a model organism for assessing the toxicity of environmental contaminates and experience reduced reproduction, growth and increased mortality with exposure to toxic substances. D.magna were exposed to concentrations of four types of functionalized AuNps.
The results showed that initial particle charge significantly impacted overall toxicity, with positively charged particles being more toxic than their negatively-charged counterparts. This could be explained by the increased cellular uptake of positively charged particles due to their high levels of attraction with cellular membrane. This creates a hole in the membrane due to the densely populated charge on the NP surface – allowing the particle to enter the intracellular matrix and continue to cause damage. Another interesting result of this study was that the smaller the particle, the higher the toxicity. This can be explained by a similar theory; after cellular uptake the smaller particles are able to cross the gut lumen of the daphnis, potentially further interacting with D. magna cells and causing damage.
The results of this study identify mechanisms for AuNP toxicity by examining NP toxicity with different charges, using an environmental relevant organism. NPs have the potential to be highly beneficial to society, but in order to minimize the environmental implications the mechanisms that govern the toxicity of NPs need to be betters elucidated. This study demonstrates how the charge and identity of a ligand can influence AuNp toxicity.
To read the full article, download your free* copy now:
Surface chemistry, charge and ligand type impact the toxicity of gold nanoparticles to Daphnia magna
Jared Bozich, Samuel E Lohse, Marco D Torelli, Catherine Murphy, Robert Hamers and Rebecca Klaper
DOI: 10.1039/C4EN00006D
*Access is free through a registered RSC account – click here to register