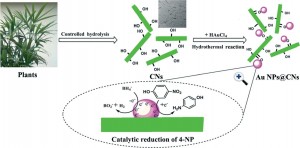
Cellulose-derived structural supports can improve the performance and the environmental credentials of gold nanoparticle catalysts. This study from the Polymer Research Institute of Sichuan University in China demonstrates the potential of this approach.
Achieving the optimum performance and stability of catalysts is of paramount importance to the development of a wide range of industrial processes and the manufacture of new products and devices. Gold nanoparticles (Au NPs) are widely used as catalysts in many chemical processes. However, these often suffer from instability owing to their large active surface area which can lead to self-aggregation. They therefore require a supporting matrix, commonly using polymeric encapsulation or support on graphene oxide or silica surfaces.
However, greener, more sustainable approaches are now being sought to improve the environmental credentials of these processes. Cellulose is the most common and abundant natural polymer. Cellulose nanocrystals (CNs) can therefore provide a possible solution as these posses the desired properties (mechanical strength, self-assembly, high specific surface area, nanometirc dispersity and high stability) to allow them to act as an effective catalyst carrier.
However the use of these have previously involved use of toxic reducing agents such as sodium borohydride or hydrazine hydride. A cleaner synthesis is required. In this study Xiaodong Wu and co workers report the first instance of a one-step, environmentally-friendly synthesis of gold NPs (Au NPs) deposited on cellulose nanocrystals and demonstrate the technical and environmental advantages of this approach.
CNs were derived from bulk cellulose using controlled acid hydrolysis of waste cotton fabric. The Au NPs were synthesised and deposited on to CNs by hydrothermal reduction of HAuCl4. Both Au NP-CN hybrids and unsupported Au NPs were prepared and their performance in the reduction of 4-nitrophenol was compared using UV-Vis spectroscopic analysis. The structure and properties of the resulting particles were characterized using transmission electron microscopy (TEM), X-Ray Diffraction (XRD) and X-Ray photoelectron spectrometric (XPS) techniques.
It was shown that the electron-rich –OH groups, abundant on their surface allow the CNs to provide the dual role of reductant and stabilizer in this process. The larger specific surface area and better dispersity of Au NPs supported on CNs meant these nano-hybrid catalysts displayed enhanced stability and superior catalytic activity than an unsupported Au NP catalyst for the reduction of 4-NP.
This approach offers a number of important advantages over previous traditional techniques. It has a lower overall cost and avoids the use of toxic or dangerous reducing, capping or dispersing agents. Furthermore this process utilises a renewable and biodegradable natural resource since the CNs can be obtained from various plant materials.
This investigation could therefore potentially pave the way to green production of bio-supported organic/inorganic nano-hybrid catalysts with possible further applications in the production of sensors, antibacterial materials and electronic devices.
To access the full article, download your free* copy by clicking the link below:
Xiaodong Wu, Canhui Lu, Zehang Zhou, Guiping Yuan, Rui Xiong and Xinxing Zhang Environ. Sci.: Nano, 2014, 1, 71-79 DOI: 10.1039/C3EN00066D
*Access is free through a registered RSC account – click here to register