Ceria nanoparticles, also known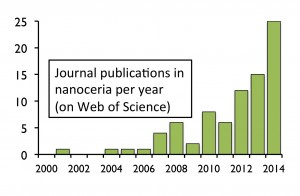 as nanoceria (cerium IV oxide, CeO2) are quickly climbing the nanotechnology popularity ladder. Ten years ago, there were hardly any academic publications using the term “nanoceria” and now there are dozens of publications per year on the subject. However, the consumer market still only has a couple of products that advertise to contain this nanomaterial.
as nanoceria (cerium IV oxide, CeO2) are quickly climbing the nanotechnology popularity ladder. Ten years ago, there were hardly any academic publications using the term “nanoceria” and now there are dozens of publications per year on the subject. However, the consumer market still only has a couple of products that advertise to contain this nanomaterial.
So, could it be that we are finally getting ahead of the curve in attempting to understand environmental impacts of this nanomaterial before it becomes widely popular?
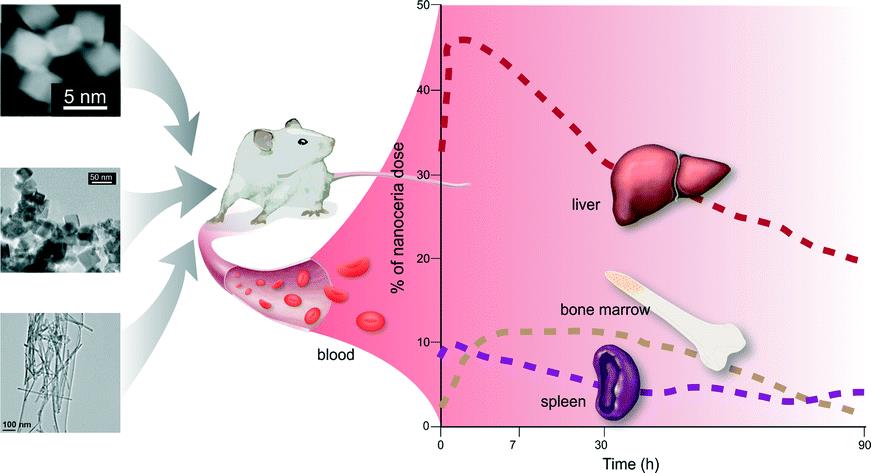
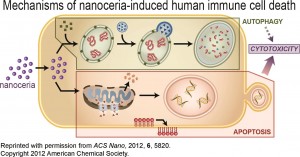
ES Nano recently published a special themed collection on this interesting nanomaterial, whose main property as a catalyst brings promise for a variety of applications. Two other Environmental Science: Nano blog posts have focused on the health effects of nanoceria and its biodistribution in rats.
 Nanoceria is a powerful catalyst because its chemical structure shows an oxygen vacancy, so oxygen atoms can move around it while oxidizing and reducing molecules in its vicinity.
Nanoceria is a powerful catalyst because its chemical structure shows an oxygen vacancy, so oxygen atoms can move around it while oxidizing and reducing molecules in its vicinity.
It absorbs reactive oxygen species (ROS), also known as free radicals, which brings a potential cosmetic and medical application. This material also absorbs UV radiation, so it might be used to replace titanium dioxide and zinc oxide in sunscreens in the future.
Since nanoceria has the potential to be a widely used in medical and cosmetic applications, it is pivotal to understand its behavior in biologically relevant environments. A recent paper by Sudipta Seal and colleagues discusses the environmental factors that can alter the properties of nanoceria and thus dictate its behavior in biological systems.
According to the authors, properties such as size, surface chemistry, surface stabilizers of nanoceria may affect its behavior in biological systems, but important issues remain to be addressed: Do slight variations in size and physico-chemical properties dictate fundamentally different behaviors? Are observed variations due to fundamentally different nanoparticles or did those particles undergo transformations? How should particles be appropriately prepared for relevant environmental and toxicology studies?
Although these general questions can be asked about a number of other nanomaterials, they are particularly relevant to nanoceria, since so little is known about this trending and promising material so far.
To access the full article, download a copy for free* by clicking the link below:
Behavior of nanoceria in biologically-relevant environments
Amit Kumar, Soumen Das, Prabhakaran Munusamy, William Self, Donald R. Baer, Dean C. Sayle and Sudipta Seal
Environ. Sci.: Nano, 2014, 1, 516-532
DOI: 10.1039/C4EN00052H
Did you like this article?
Find out more about Marina in her first Environmental Science: Nano blog article on carbon nanotubes.
* Access is free through a registered RSC account – click here to register