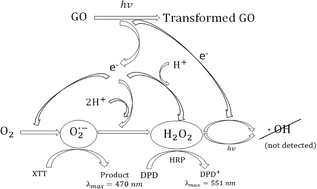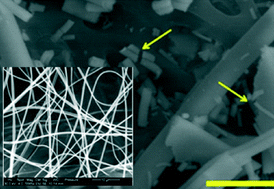
Alumina (Al2O3) nanofibers have potential applications as catalyst support structures, reaction substrates, filtrations devices and sensors as a result of their high thermal stability. On the other hand, the fibrous nature of these materials calls for extra caution because of their potency to cause pulmonary diseases.
Fiber respirability and durability are among the dominant factors contributing towards the potential toxicity. The aerodynamic diameter controls the respirability while dissolution is related to the durability. A fiber is considered bio-durable if the rate at which it dissolves via chemical dissolution is slower than the rate of physical removal by the lung by mechanical action.
Therefore, Hyeon Ung Shin, Aleksandr B. Stefaniak, Nenad Stojilovic and George G. Chase from University of Akron, National Institute for Occupational Safety and Health and University of Wisconsin Oshkosh has investigated the dissolution of electrospun Al2O3 nanofibers in human artificial lung fluid and free radical generation to determine the influence of physicochemical properties.
These fibers were prepared using different thermal treatments and were characterized extensively for size, surface morphology, crystal structure and surface area. Then dissolution was measured by incubating the fibers in serum ultrafiltrate and phagolysosomal simulant fluid and analyzing the supernatant using ICP-OES at different time intervals. Dissolution rates were calculated assuming constant dissolution velocity:
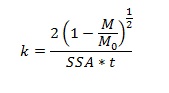
Where (1-M/Mo) is the mass fraction of material dissolved, t is the time (days), SSA is the specific surface area (cm2g-1) and k is the chemical dissolution rate constant. The free radical generation was probed using electron spin resonance spectroscopy (ESR).
The study has shown no effect of physicochemical properties on the Al2O3 dissolution in artificial human lung fluid indicating the differences in the heat treatments does not affect the solubility within lungs. However, greater dissolution rates were observed for the samples with higher heating ramp rates even though their physicochemical properties were similar. No measurable levels of free radicals were generated by these alumina nanofibers.
To access the full article, download a copy for free* by clicking the link below.
Comparative dissolution of electrospun Al2O3 nanofibres in artificial human lung fluids
Hyeon Ung Shin, Aleksandr B. Stefaniak, Nenad Stojilovic and George G. Chase
Environ. Sci: Nano, 2015, 2, 251-261
DOI: 10.1039/C5EN00033E
—————-

About the webwriter
Imali Mudunkotuwa is a Postdoctoral Scholar and Research Assistant at The University of Iowa. She is interested in nanoscience, physical and surface chemistry. You can find more articles by Imali in her author archive .
—————-
* Access is free until the 06/10/2015 through a registered RSC account