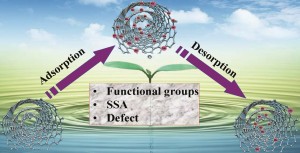
Carbon nanotubes (CNTs) are promising nanomaterials because of their many interesting properties: CNTs are flexible yet super strong and practically impossible to break. Depending on their atomic arrangement, CNTs can be semiconductors or very strong conductors of electricity. When it comes to transporting heat, this amazing material can move it very quickly along its length, while behaving as an insulator from side to side. CNTs can also be doped or decorated with different chemical elements, compounds, or nanoparticles and serve as a transport carrier for those materials. For these reasons, along with many others, CNTs are being developed for a myriad of applications, ranging from electronics to drug delivery. Because on this increasing interest in applications of CNTs, we can expect their global production to grow over time, which may lead to an increase in the potential for CNTs to be released into the environment or to be put into contact with people.
Jie Li and colleagues explored the possibility that CNTs may serve as a “Trojan Horse” by carrying heavy metal ions that were incorporated onto their surface and then releasing those ions when they reach the environment. Imagine the giant Greek wooden horse, arriving the gates of the city of Troy, full of soldiers in its belly. Now, scratch that and imagine a carbon nanotube arriving in your local river, its back teeming with heavy metals. Will these metals dismount the CNTs and impact the wildlife of your local river? Will they go through the local treatment plant and arrive in your home, via your tap water? Or will these metals stay forever stuck onto the CNTs and not cause any harm?
To answer this question, researchers from China studied different types of CNTs with a variety of metal ions: copper, hexavalent chromium (a known carcinogen), and arsenic (a known toxic chemical).
They found that the absorption and desorption of heavy metals from CNTs do not occur at the same pace. In practical terms, the metal ions come off the CNTs slower than the rate at which they are put onto the CNTs. When comparing different types of CNTs, they found that multi-walled CNTs and double-walled CNTs have a higher capacity to carry arsenic and chromium cations than single walled CNTs or oxidized CNTs. For copper (an anion), they observed exactly the opposite.
The sorption of heavy metal ions onto the surface of CNTs is a reversible process.Therefore it is possible that once CNTs enter the environment, they release the metals which they were carrying. The higher the concentration of metals, the more reversible this process might be. The release of metals from CNTs occurs differently for negatively versus positively charged ionic metals and varies greatly among different types of CNTs. This means that we cannot predict what will happen to CNTs (and the metals they carry) in the environment without understanding their structure and knowing exactly what types of chemicals are sorbed onto their surface.
To access the full article, download a copy for free* by clicking the link below.
* Access is free through a registered RSC account – click here to register
About the webwriter
 Marina Vance is a research scientist at Virginia Tech and associate director of the Virginia Tech Center for Sustainable Nanotechnology Her work focuses on people’s exposure to nanomaterials.
Marina Vance is a research scientist at Virginia Tech and associate director of the Virginia Tech Center for Sustainable Nanotechnology Her work focuses on people’s exposure to nanomaterials.
Follow her on twitter: @marinavance