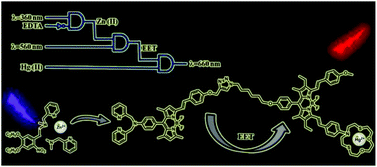
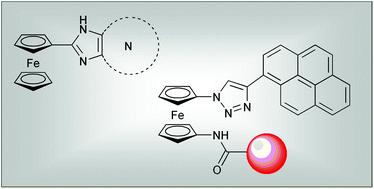
Modular logic gates: cascading independent logic gates via metal ion signals
Esra Tanriverdi Ecik, Ahmet Atilgan, Ruslan Guliyev, T. Bilal Uyar, Aysegul Gumus and Engin U. Akkaya
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C3DT52375F
Free to access until 16th December.
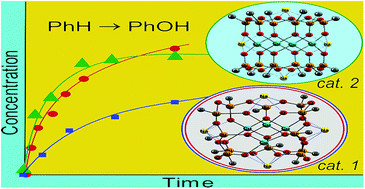
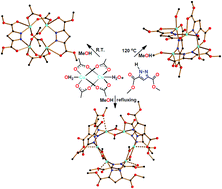
Solvent-controlled synthesis of tetranuclear cage-like copper(II) silsesquioxanes. Remarkable features of the cage structures and their high catalytic activity in oxidation with peroxides
Marina S. Dronova, Alexey N. Bilyachenko, Alexey I. Yalymov, Yuriy N. Kozlov, Lidia S. Shul’pina, Alexander A. Korlyukov, Dmitry E. Arkhipov, Mikhail M. Levitsky, Elena S. Shubina and Georgiy B. Shul’pin
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C3DT52508B
Free to access until 16th December.
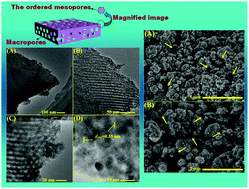
A floating macro/mesoporous crystalline anatase TiO2 ceramic with enhanced photocatalytic performance for recalcitrant wastewater degradation
Zipeng Xing, Wei Zhou, Fan Du, Yang Qu, Guohui Tian, Kai Pan, Chungui Tian and Honggang Fu
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C3DT52433G
Free to access until 16th December.
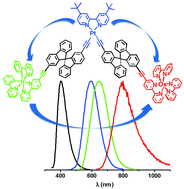
Fac and mer isomers of Ru(II) tris(pyrazolyl-pyridine) complexes as models for the vertices of coordination cages: structural characterisation and hydrogen-bonding characteristics
Alexander J. Metherell, William Cullen, Andrew Stephenson, Christopher A. Hunter and Michael D. Ward
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52479E
Free to access until 16th December.
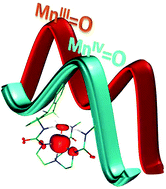
![[double bond, length as m-dash]](http://www.rsc.org/images/entities/h2_char_e001.gif) O complexes: how important is the oxo ligand basicity in the C–H activation step?
O complexes: how important is the oxo ligand basicity in the C–H activation step?Madhavan Jaccob, Azaj Ansari, Bhawana Pandey and Gopalan Rajaraman
Dalton Trans., 2013,42, 16518-16526
DOI: 10.1039/C3DT52290C
Free to access until 16th December.
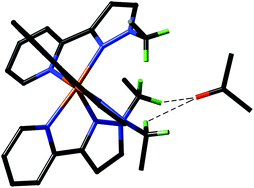
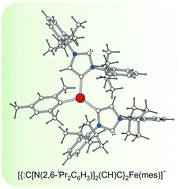
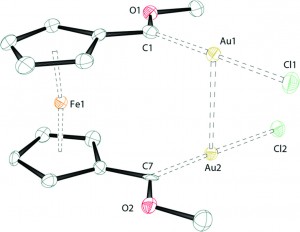
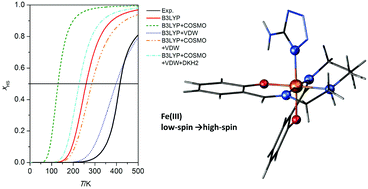
Iron(II) complexes of ditopic carbanionic carbenes
Rebecca A. Musgrave, Robert S. P. Turbervill, Mark Irwin, Radovan Herchel and Jose M. Goicoechea
Dalton Trans., 2014, Advance Article
DOI: 10.1039/C3DT52638K
Free to access until 16th December.