Posted on behalf of Lewis Downie, web writer for Dalton Transactions
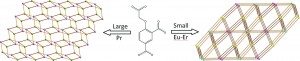
Metal organic frameworks (MOFs) can show a range of structures and properties and have proved an interesting avenue for contemporary research into areas such as gas storage and heterogeneous catalysis. These applications have tended to use transition metals and as such the metal coordination is typically four or six-fold. The use of lanthanide ions increases the variety of coordination numbers and also gives access to more properties which make use of the electronic structure of the lanthanides: namely magnetic and luminescent behaviours. If the MOF consists not only of lanthanides but also conjugated ligands then it is possible to increase electronic communication between metal centres and also electromagnetically sensitise them. With this in mind, Zhao et al. have hydrothermally synthesised a range of MOFs based on the trivalent lanthanide metal centres Pr, Eu, Gd, Tb, Dy, Ho and Er and the organic linker 4-(carboxymethoxy)isophthalic acid.
The extraordinary flexibility of the metal coordination site is quite exceptional, and due to changing cation size there is the appearance of two different structure types. For the largest cation, Pr3+, a 2D framework is formed with Pr3+ showing a coordination sphere of ten. This is composed of a number of varying ligands; three bidentate and two monodentate carboxyl groups from 4-(carboxymethoxy)isophthalic acid and two coordinating water molecules. This compound is very stable for a MOF with thermogravimetric analysis (TGA) indicating decomposition at 400° C. As the lanthanide increases in size the coordination drops to eight; this is found for all the other lanthanide materials synthesised and they are all isostructural. The phase found for the smaller lanthanides is significantly different however – in this case the structure is found to be a 3D framework. There are also three crystallographically distinct Ln3+ cations showing a range of binding types as before. TGA measurements again suggest that the material is particularly temperature stable.
As previously mentioned MOFs which contain lanthanides can show some interesting behaviours and the examples reported in this paper are no exception. Examples of luminescence are found in the frameworks containing Eu3+ (red), Tb3+(green) and Dy3+ (yellow). The magnetic properties of the materials were also investigated. Measurements indicate that they may have weak antiferromagnetic interactions, however, as the effects are quite small this is difficult to resolve over the behaviour of the free Ln3+ ions.
Find out more:
Several (4,4)- and (5,6,8)-connected lanthanide–organic frameworks: structures, luminescence and magnetic properties
Xiao-Qing Zhao, Xu-Hui Liu and Bin Zhao
Dalton Trans., 2013, Advance Article
DOI: 10.1039/C3DT51769A, Paper
 Lewis Downie has wide ranging interests in the chemical sciences but has a background in functional materials. His main research focus is the investigation of these materials using crystallographic techniques. He is currently a postdoctoral research assistant at the University of St Andrews, U.K.
Lewis Downie has wide ranging interests in the chemical sciences but has a background in functional materials. His main research focus is the investigation of these materials using crystallographic techniques. He is currently a postdoctoral research assistant at the University of St Andrews, U.K.