Toshihiro Ihara and colleagues at Kumamoto University demonstrate a quick reversible photocircularization of an anthracene-modified oligodeoxyribonucleotide conjugate and provide an example of its analytical application.
‘ The oligonucleotide conjugate was reversibly circularized through photodimerization of the antracenes attached on both ends. This process would be potentially useful as a probe reaction with high specificity and sensitivity’ say the authors on the description of their cover.
Read the article free to access until the end of November
Reversible circularization of an anthracene-modified DNA conjugate through bimolecular triplex formation and its analytical application
Pelin Arslan, Akinori Jyo and Toshihiro Ihara
Org. Biomol. Chem., 2010, 8, 4843-4848
DOI: 10.1039/C0OB00282H












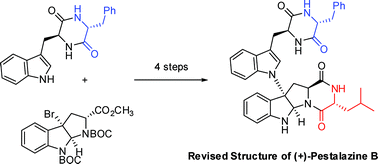
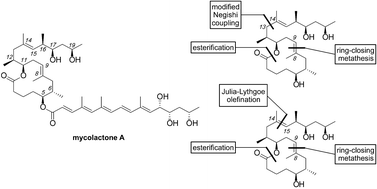 Synthetic studies on the mycolactone core
Synthetic studies on the mycolactone core 
 Hans-Joachim Knölkner and colleagues reveal the link between this beautiful church in Dresden and β-isocomene: silicon
Hans-Joachim Knölkner and colleagues reveal the link between this beautiful church in Dresden and β-isocomene: silicon This colourful and arty cover by Chunling Fu, Shengming Ma and colleagues shows the mechanism of a highly regio- and stereo- selective hydration of 1,2-allenylic sulfoxides.
This colourful and arty cover by Chunling Fu, Shengming Ma and colleagues shows the mechanism of a highly regio- and stereo- selective hydration of 1,2-allenylic sulfoxides.