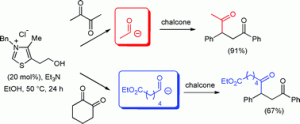
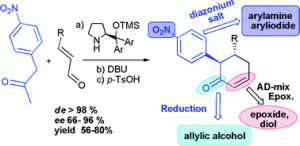
Alison R. Hardin Narayan and Richmond Sarpong have investigated the fundamental reactivity of indolizinones, relatively unexplored 6,5-N-fused bicycles. They find that these bicycles have excellent ability to transfer stereochemistry from the ring fusion position to other stereocenters, as well as readily undergoing Diels-Alder cycloadditions and selective hydrogenation, among other reactions.
Alison R. Hardin Narayan and Richmond Sarpong have investigated the fundamental reactivity of indolizinones, relatively unexplored 6,5-N-fused bicycles. They find that these bicycles have excellent ability to transfer stereochemistry from the ring fusion position to other stereocenters, as well as readily undergoing Diels-Alder cycloadditions and selective hydrogenation, among other reactions.
To read more on the potential applications of indolizinones download the article now – it’s free to access for the next four weeks:
Indolizinones as synthetic scaffolds: fundamental reactivity and the relay of stereochemical information
Alison R. Hardin Narayan and Richmond Sarpong
DOI: 10.1039/C1OB06423A











![GA[7]](https://blogs.rsc.org/md/files/2011/11/GA7-300x128.gif)
![GA[8]](https://blogs.rsc.org/ob/files/2011/11/GA8-300x82.gif)