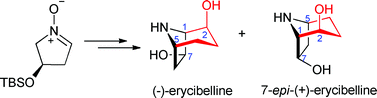
In a quest for bioactive metabolites from Chinese medicinal plants, Yue-Wei Guo et al. have isolated and characterised a number of novel and exciting natural products from Toona ciliata var. Pubescens, a tall timber tree which can be found widely in south China.
The Toona species have previously led to the isolation of limonoids and protolimonoids. These complex molecules, bearing an elaborate polycyclic skeleton, represent challenging targets for total synthesis and are particularly attractive due to their range of biological activities.
In this paper, the team of collaborative researchers from China and Hungary have isolated nine new limonoids, three of them possessing unprecedented carbon skeletons, along with 5 known analogs. Using advanced NMR techniques, a solid-state TDDFT ECD approach and X-ray analysis, a complete evaluation of their relative and absolute configuration was determined. Biological activities were found for Toonapubesin G (7), showing promising inhibitory activity against the aggressive tumor cell line CDC25B and compound 8a showed significant cell protecting activity against H2O2-induced SH-SY5Y cell damage.
![GA[1]](https://blogs.rsc.org/np/files/2011/10/GA1.gif)
This article will be FREE to access for the next 4 weeks:
Protolimonoids and norlimonoids from the stem bark of Toona ciliata var. pubescens
Jian-Rong Wang, Hai-Li Liu, Tibor Kurtán, Attila Mándi, Sándor Antus, Jia Li, Hai-Yan Zhang and Yue-Wei Guo
Org. Biomol. Chem., 2011, Advance Article
DOI: 10.1039/C1OB06150J, Paper
Interested in Natural Products? Why not also take a look at our sister journal Natural Product Reports and keep up-to-date with high impact, critical reviews in natural products research.
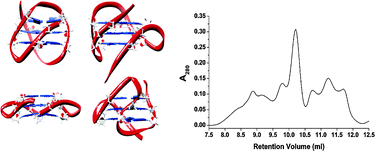
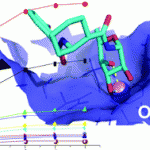 The binding of two α-fucosylamide-based mimics of LewisX to DC-SIGN ECD (Dendritic Cell-Specific ICAM-3 Grabbing Nonintegrin extracellular domain) using STD NMR and docking studies has been investigated by Pedro M. Nieto and coworkers. The mimics are shown to bind in a similar way to LewisX, through the fructose moiety and in a multi-modal fashion, but with increased affinity due to the aromatic moiety.
The binding of two α-fucosylamide-based mimics of LewisX to DC-SIGN ECD (Dendritic Cell-Specific ICAM-3 Grabbing Nonintegrin extracellular domain) using STD NMR and docking studies has been investigated by Pedro M. Nieto and coworkers. The mimics are shown to bind in a similar way to LewisX, through the fructose moiety and in a multi-modal fashion, but with increased affinity due to the aromatic moiety.










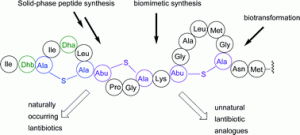


![GA[7]](https://blogs.rsc.org/ob/files/2011/10/GA7.gif)
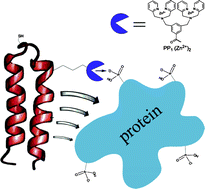
![GA[1]](https://blogs.rsc.org/np/files/2011/10/GA1.gif)